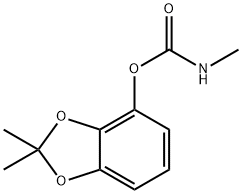
BENDIOCARB
- CAS No.
- 22781-23-3
- Chemical Name:
- BENDIOCARB
- Synonyms
- Fuam;FICAM;Sedox;tatoo;DYCARB;GARVOX;nc6897;Niomil;Rotate;Tattoo
- CBNumber:
- CB0203933
- Molecular Formula:
- C11H13NO4
- Molecular Weight:
- 223.23
- MDL Number:
- MFCD00078629
- MOL File:
- 22781-23-3.mol
| Melting point | 129-130° |
|---|---|
| Boiling point | 364.51°C (rough estimate) |
| Density | 1.2500 |
| vapor pressure | 4.6 x 10-3 Pa (25 °C) |
| refractive index | 1.4960 (estimate) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| solubility | DMF: 30 mg/ml,DMSO: 30 mg/ml,DMSO:PBS (pH 7.2) (1:9): 0.1 mg/ml,Ethanol: 5 mg/ml |
| form | Powder/Solid |
| Water Solubility | 280 mg l-1 (20 °C) |
| pka | 11.98±0.46(Predicted) |
| color | White |
| Merck | 13,1033 |
| BRN | 1315404 |
| InChIKey | XEGGRYVFLWGFHI-UHFFFAOYSA-N |
| CAS DataBase Reference | 22781-23-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | QFH0ZU0A5U |
| NIST Chemistry Reference | Bendiocarb(22781-23-3) |
| EPA Substance Registry System | Bendiocarb (22781-23-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H311+H331-H410 | |||||||||
| Precautionary statements | P261-P273-P280-P301+P310-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 21-23/25-50/53 | |||||||||
| Safety Statements | 22-36/37-45-60-61 | |||||||||
| RIDADR | 2757 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FC1140000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29329990 | |||||||||
| Toxicity | LC50 (96-hour) for rainbow trout 1.55 mg/L (Hartley and Kidd, 1987); acute oral LD50 for rats 40–156 mg/kg (Hartley and Kidd, 1987). | |||||||||
| NFPA 704 |
|
BENDIOCARB Chemical Properties,Uses,Production
Description
Bendiocarb belongs to a kind of carbamate insecticide. It can be used in homes, industrial plants and food storage sites for the treatment of various kinds of insects including mosquitoes, flies, wasps, ants, fleas, cockroaches, spider, silverfish, ticks and other pests in homes, industrial plants, and food storage sites. In agriculture, it is used for the treatment of a variety of insects such as beetles, aphids, mites and caterpillars. Bendiocarb can block the activity of some enzymes necessary for the normal nerve transmission, further affecting the normal operation of the insects’ nervous system. It can kill the pests through either contact or ingestion. However, its applications have been cancelled in some countries including the United States due to its toxicity effects on Human beings and other animals including birds and fishes.
References
https://en.wikipedia.org/wiki/Bendiocarb
http://npic.orst.edu/factsheets/bendiogen.pdf
http://pmep.cce.cornell.edu/profiles/extoxnet/24d-captan/bendiocarb-ext.html
Description
Bendiocarb is an odourless, non-corrosive white crystalline solid. Bendiocarb is a carbamate ester. Carbamates are chemically similar to but more reactive than amides. Like amides, they form polymers such as polyurethane resins. Some of the formulations of bendiocarb are classified as general use pesticides (GUP), while Turcam and its 2.5 G formulation have been classified as restricted use pesticides (RUP). Bendiocarb is stable under normal temperatures and pressures, but should not be mixed with alkaline preparations. Thermal decomposition products may include toxic oxides of nitrogen. It is noncorrosive. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidising acids, peroxides, and hydroperoxides are incompatible with carbamates. Bendiocarb as a carbamate insecticide is effective against a wide range of insects that cause nuisance and act as disease vectors. It is used to control mosquitoes, flies, wasps, ants, fleas, cockroaches, silverfish, ticks, and other pests in homes, industrial plants, and food storage sites. Bendiocarb is also used as a seed treatment on sugar beets and maize and against snails and slugs. Pesticides containing bendiocarb are formulated as dusts, granules, ultra-low volume sprays, and wettable powders. It gets hydrolysed rapidly in alkali media and slows under acid and neutral conditions. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates.
Chemical Properties
Bendiocarb is a white odorless crystalline powder.
Chemical Properties
Bendiocarb is an odorless, white crystalline solid. Some of the formulations of bendiocarb are classii ed as a GUP, while Turcam and its 2.5 G formulation have been classii ed as a RUP. Bendiocarb is stable under normal temperatures and pressures, but should not be mixed with alkaline preparations. Thermal decomposition products may include toxic oxides of nitrogen. It is non-corrosive. Bendiocarb as a carbamate insecticide is effective against a wide range of insects that cause nuisance and act as disease vectors. It is used to control mosquitoes, l ies, wasps, ants, l eas, cockroaches, silveri sh, ticks, and other pests in homes, industrial plants, and food storage sites. In agriculture, it is used against a vari- ety of insects, especially those in the soil. Bendiocarb is also used as a seed treatment on sugar beets and maize and against snails and slugs. Pesticides containing bendiocarb are formulated as dusts, granules, ultra-low volume sprays, and as wettable powders
Uses
Contact insecticide.
Uses
Contact insecticide used to control beetles, wireworms, flies, wasps and mosquitoes in beets and maize.
Uses
Bendiocarb is a contact and ingested insecticide with some systemic activity in crop plants. It is active against many public health, industrial and storage pests such as Formicidae, Blattodae, Culicidae, Muscidae and Siphonaptera.
Uses
Bendiocarb is a benzodioxolyl carbamate derivative. Bendiocarb is commonly used as an insecticide in agriculture against a wide range of pests and insects. Bendiocarb is one of the insecticides recomm ended by world health organization (WHO) for use in malaria control.
Definition
ChEBI: Bendiocarb is a carbamate ester and a member of benzodioxoles. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, a carbamate insecticide and an agrochemical. It is functionally related to a methylcarbamic acid.
General Description
White solid. Melting point 265°F (129-30°C). Insoluble in water. Used as a contact insecticide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
BENDIOCARB is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Hazard
Poison by ingestion, skin absorption.
Health Hazard
Highly toxic by all routes of exposure; oralmedian lethal doses in rats, mice, guineapigs, and rabbits range at 30–50 mg/kg;cholinesterase inhibitor; toxic symptomsinclude increased salivation, lacrimation,sweating, blurred vision, twitching of muscle, tremor, weakness, vomiting, abdominalcramps, and diarrhea; high doses can causedeath; absorption through skin may be slow.
LD50 oral (rat): 40 mg/kg
LD50 oral (rabbit): 35 mg/kg
LD50 skin (rat): ~600 mg/kg.
Health Hazard
Bendiocarb is moderately toxic if ingested or absorbed through the skin. Absorption through the skin is the most likely route of exposure. Bendiocarb is absorbed through all the normal routes (oral, dermal, and inhalation) of exposure, but dermal absorption is especially rapid. Carbamates are generally excreted rapidly and do not accumulate in mammalian tissue. If exposure does not continue, cholinesterase inhibition and its symptoms reverse rapidly. In non-fatal cases, the illness generally lasts less than 24 h. Bendiocarb is moderately toxic to birds. The LD50 in mallard ducks and quail is 3.1 and 19 mg/kg, respectively. Bendiocarb is moderately to highly toxic to i sh. The LC50 (96 h) for bendiocarb in rainbow trout is 1.55 mg/L. Bendiocarb is a mild irritant to the skin and eyes. Symptoms of bendiocarb poisoning include, but are not limited to, weakness, blurred vision, headache, nausea, abdominal cramps, chest discomfort, constriction of pupils, sweating, muscle tremors, and decreased pulse. Prolonged exposures to high concentrations of bendiocarb cause severe poisoning with symptoms of twitching, giddiness, confusion, muscle incoordination, slurred speech, low blood pressure, heart irregularities, and loss of rel exes may also be experienced. Death can result from respiratory arrest, paralysis of the muscles of the respiratory system, intense constriction of the openings of the lung, or all three. In one case of exposure while applying bendiocarb, the victim experienced symptoms of severe headache, vomiting, and excessive salivation, and his cholinesterase level was depressed by 63%. He recovered from these symptoms in less than 3 h with no medical treatment and his cholinesterase level returned to normal within 24 h. In another case, poisoning occurred when an appli- cator, who was not wearing protective equipment, attempted to clean contaminated equip- ment. The victim experienced nausea, vomiting, incoordination, pain in his arms, hands, and legs, muscle spasms, and breathing difi culties. Bendiocarb is readily absorbed by the gastrointestinal tract and is rapidly metabolized.
Safety Profile
Poison by ingestion. Moderately toxic by skin contact. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES.
Potential Exposure
A potential danger to those involved in the manufacture, formulation and application of this carbamate insecticide which is used against household pests, in agriculture in seed treatment, and as a foliar spray.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Shampoo hair. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Soil. Though no products were identified, the reported half-life in soil is several days
to a few weeks (Hartley and Kidd, 1987). When soil containing bendiocarb was incubated
for 4 weeks, the amount remaining was 30.4–47.2% of the applied amount. After one
application, the amount of bendiocarb recovered was only 0.9–1.3% (Racke and Coates,
1988). Biodegradation of bendiocarb in soil was enhanced if the soil was pretreated with
carbofuran or trimethacarb (Dzantor and Felsot, 1989).
Plant. At planting, bendiocarb was applied at a rate of 1.7 kg/ha. During harvest (110
days following planting), the residues of bendiocarb found in corn (leaves and stems),
husks, cobs and kernels were 12, 5, 4 and 3 ppb, respectively. At an app
Chemical/Physical. Bendiocarb is stable to light but in water hydrolyzes to 2,3-
isopropylidenedioxyphenol, methylamine and carbon dioxide. The hydrolysis half-life at
pH 7 and 25°C is 4 days (Worthing and Hance, 1991).
Metabolic pathway
Pathways of bendiocarb metabolism in mammals include hydrolysis to the benzodioxol-4-ol, hydroxylation of the phenyl ring, hydroxylation at the N-methyl moiety and conjugation. Extensive pathways reported for the structurally related carbofuran are not reported for bendiocarb partly because hydroxylation and subsequent oxidation and conjugation at the 3-position is not possible in bendiocarb although it is a major pathway for carbofuran. No domation is available for the metabolism of bendiocarb in plants.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Color Code—Red:Flammability Hazard: Store in a flammable liquid storagearea or approved cabinet away from ignition sources andcorrosive and reactive materials. Color Code—White:Corrosive or Contact Hazard; Store separately in a corrosion-resistant location. Color Code—Green: General storagemay be used. Prior to working with Bendiocarb you shouldbe trained on its proper handling and storage. Store intightly closed containers in a cool, well-ventilated areaaway from food, fertilizers, other pesticides, flammablematerials, and sources of heat and flame.
Shipping
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1—Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Degradation
Bendiocarb undergoes base-catalysed hydrolysis but is more stable in neutral or acidic conditions. The products of hydrolysis are 2,2-dimethyl- 1,3-benzodioxol-4-ol(2), methylamine and CO2. Its DT50 at ph 7 and 25 °C is 4 days (PM).
Incompatibilities
Carbamates are incompatible with reducing agents, strong acids, oxidizing acids, peroxides, and bases. Contact with active metals or nitrides cause the 360 Bendiocarb release of flammable, and potentially explosive, hydrogen gas. May react violently with bromine, ketones. Incompatible with azo dyes, caustics, ammonia, amines, boranes, hydrazines, strong oxidizers. Keep away from flammable materials and sources of heat and flame.
Waste Disposal
Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Dispose in accordance with 40CFR165 recommendations for the disposal of pesticides and pesticide containers.
Precautions
The preliminary risk assessment of the US EPA showed that applicators, including home owners, were at risk when mixing or applying the pesticide. Thus, bendiocarb is no longer sold in the United States. All bendiocarb-containing products in the United States recently had their registrations cancelled due to concerns over exposure of those applying the products. In the UK, however, bendiocarb is still registered as a biocide and amateurs can use it even though it lacks proper warning labels. In view of this, bendiocarb should be purchased and used only by certii ed and trained applicators. Like all RUPs, management must be done only by trained and certii ed workers.