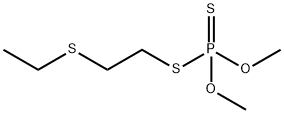
THIOMETON
- CAS No.
- 640-15-3
- Chemical Name:
- THIOMETON
- Synonyms
- m81;M 81;ebicid;ekatim;Medrin;san230;Veltin;EKATIN;Nimeton;SAN 230
- CBNumber:
- CB0731014
- Molecular Formula:
- C6H15O2PS3
- Molecular Weight:
- 246.35
- MDL Number:
- MFCD01310440
- MOL File:
- 640-15-3.mol
| Melting point | <25℃ |
|---|---|
| Boiling point | 110°C |
| Density | 1.209 g/cm3 (20℃) |
| vapor pressure | 3.99×10-2 Pa (20 °C) |
| refractive index | 1.568 (589.3 nm 20℃) |
| Flash point | 70 °C |
| storage temp. | 2-8°C |
| solubility | soluble in No data available |
| Water Solubility | 200 mg l-1(25 °C) |
| form | liquid |
| CAS DataBase Reference | 640-15-3 |
| EWG's Food Scores | 1 |
| FDA UNII | YDI0MXQ7IR |
| EPA Substance Registry System | Thiometon (640-15-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H312 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P280-P302+P352-P312-P322-P363-P501 |
| Hazard Codes | T,N |
| Risk Statements | 21-25-20/21-66-65-51/53-40 |
| Safety Statements | 36/37-45-62-61 |
| RIDADR | 3018 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
THIOMETON Chemical Properties,Uses,Production
Description
Thiometon is the thion (P=S) analog of demeton-Smethyl. It is a colorless oil, bp 104 ?C/0.3 mm Hg, vp 39.9 mPa (20 ?C). The water solubility is 200 mg/L (27 ?C). It is highly soluble inmost organic solvents except alkanes. Log Kow = 3.15. It is hydrolyzed in alkaline and acidic media; DT50 (25 ?C) values at pH 3, 6, and 9 are 25, 27, and 17 d, respectively.
Uses
Thiometon is used to control sucking insects and mites on a wide range of crops.
Definition
ChEBI: Thiometon is an organic thiophosphate, an organothiophosphate insecticide and an organosulfur compound. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide and an agrochemical. It is functionally related to a 2-(ethylsulfanyl)ethanethiol.
Safety Profile
Poison by ingestion, skin contact, inhalation, and intravenous routes. Mutation data reported. A skin and severe eye irritant. A cholinesterase inhibitor. When heated to decomposition it emits very toxic fumes of POx and SOx. See also ESTERS and PARATHION
Metabolic pathway
Thiometon is the thion (P=S) analogue of demeton-S-methyl. Consequently, many of the biotransformations of hometon are similar to those of demeton-S-methyl to which it is oxidised in animals. It is also the dimethyl analogue of disulfoton. However, thiooxidation in soil and plants appears to be more facile than oxidative desulfuration to the oxon so that its metabolism is analogous to phorate and disulfoton in that the compound is first thiooxidised to thiometon sulfoxide and sulfone which are oxidatively desulfurated to oxydemeton-S-methyl and demeton-S-methylsulfon respectively.
Degradation
Thiometon is more easily hydrolysed in alkaline media than in acid. The DT50 values at pH 3,6 and 9 were 25,27 and 17 days at 25 °C (PM). The rates of hydrolysis at pH values 5.7 and 8.5 for thiometon, disulfoton, demeton-S-methyl and diazinon catalysed by alumina and three different forms of iron oxide were determined by Dannenberg and I'ehkonen (1998). Products were analysed by GC-MS. In the presence of oxygen, the expected thiol product of hydrolysis of the former three compounds, 2- ethylthioethanethiol(2), was not observed at any stage during the course of the reaction. Instead it was found to dimerise to the disulfide (3). Under nitrogen, the main product of disulfoton hydrolysis was an ethylated thiol which was probably formed via nucleophilic attack of the thiol (2) on disulfoton, although the experiment was not performed with thiometon itself.
Toxicity evaluation
The acute oral LD50 for rats is 70–120 mg/kg. Thiometon is metabolized oxidatively in plants, forming demeton-S-methyl sulfoxide and sulfone, which are the active principles.