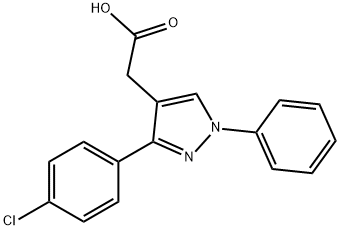
lonazolac
- CAS No.
- 53808-88-1
- Chemical Name:
- lonazolac
- Synonyms
- lonazolac;LONAZOLAC CALCIUM;3-(4-Chlorophenyl)-1-phenyl-1H-pyrazole-4-acetic acid;1H-Pyrazole-4-acetic acid, 3-(4-chlorophenyl)-1-phenyl-;2-[3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl]acetic acid;anti-inflammatory,antirheumatic,non-acidic,Inhibitor,inhibit,Lonazolac
- CBNumber:
- CB1936094
- Molecular Formula:
- C17H13ClN2O2
- Molecular Weight:
- 312.755
- MDL Number:
- MFCD00866046
- MOL File:
- 53808-88-1.mol
| Melting point | 150-151° |
|---|---|
| pka | 4.3(at 25℃) |
| FDA UNII | 13097143QI |
| ATC code | M01AB09 |
lonazolac price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0015859 | [3-(4-CHLOROPHENYL)-1-PHENYL-1H-PYRAZOL-4-YL]ACETIC ACID 95.00% | 53808-88-1 | 1G | $453.6 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0015859 | [3-(4-CHLOROPHENYL)-1-PHENYL-1H-PYRAZOL-4-YL]ACETIC ACID 95.00% | 53808-88-1 | 2.5G | $1227.82 | 2021-12-16 | Buy |
lonazolac Chemical Properties,Uses,Production
Originator
Irriten,Tosse,W. Germany,1981
Definition
ChEBI: A monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is replaced by a 3-(4-chlorophenyl)-1-phenylpyrazol-4-yl group.
Manufacturing Process
17.6 g 1-phenyl-3-(p-chlorophenyl)-pyrazol-4-acetonitrile and 180 ml 25% aqueous hydrochloric acid were mixed and heated to the boiling temperature under reflux for 6 hours. To the mixture was then added dropwise concentrated aqueous sodium hydroxide until the pH of the mixture reached a value in the range from 3 to 5. The free pyrazol-4-acetic acid precipitated thereby was filtered off, redissolved in dilute aqueous sodium hydroxide, the solution cleared by treatment with activated carbon, and the pyrazol-4-acetic acid precipitated by acidifying the solution by the addition of dilute mineral acid, sulfuric acid. The filtered acid was crystallized from a mixture of ethanol and water. 17.1 g 1-phenyl-3-(p-chlorophenyl)pyrazol-4-acetic acid, melting at 148°C to 150°C, were obtained, representing a yield of 91%.
Therapeutic Function
Antiinflammatory
lonazolac Preparation Products And Raw materials
lonazolac Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Changzhou Hopschain Chemical Co.,Ltd. | 0519-85528066 13775048983 | sales@hopschem.com | China | 31557 | 55 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Suzhou Zhixin Biotechnology Co., Ltd. | 0512-65118909 15162312715 | sales@szzxbio.com | China | 2999 | 58 |
| Nantong QuanYi Biotechnology Co., Ltd | 0513-66337626 18051384581 | sales@chemhifuture.com | China | 4030 | 58 |
| Yantai Lianhua Technology Co., Ltd | 15552437730 | 1701654643@qq.com | China | 11399 | 58 |
| Nanjing Shizhou Biology Technology Co.,Ltd | 025-85560043 15850508050 | cindy.huang@synzest.com | China | 12007 | 58 |