STRONTIUM
- CAS No.
- 7440-24-6
- Chemical Name:
- STRONTIUM
- Synonyms
- STRONTIUM;Aids072432;Aids-072432;strontium(2+);strontium atom;STRONTIUM METAL;Strontium pieces;Strontium, 99.9%;Strontium Powder;Strontium, Chunks
- CBNumber:
- CB2350213
- Molecular Formula:
- Sr
Lewis structure
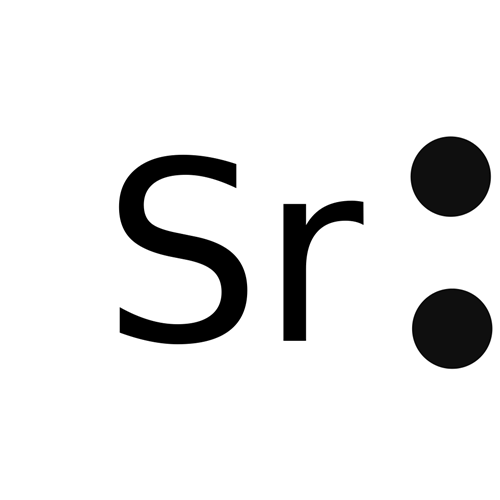
- Molecular Weight:
- 87.62
- MDL Number:
- MFCD00134060
- MOL File:
- 7440-24-6.mol
| Melting point | 757 °C (lit.) |
|---|---|
| Boiling point | 1384 °C (lit.) |
| Density | 2.6 g/mL at 25 °C (lit.) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | random pieces |
| color | White to pale yellow |
| Specific Gravity | 2.54 |
| Resistivity | 23 μΩ-cm, 20°C |
| Water Solubility | reacts quickly with H2O; soluble alcohol [HAW93] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,8915 |
| Exposure limits |
ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-24-6(CAS DataBase Reference) |
| EWG's Food Scores | 1-4 |
| NCI Dictionary of Cancer Terms | strontium |
| FDA UNII | YZS2RPE8LE |
| EPA Substance Registry System | Strontium (7440-24-6) |
| Bulk Modulus | 11.61 GPa |
|---|
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H260-H315 | |||||||||
| Precautionary statements | P231+P232-P302+P352 | |||||||||
| Hazard Codes | F,Xi,T | |||||||||
| Risk Statements | 37/38-41-38-14-11-36/38-34-23/24/25-36-14/15 | |||||||||
| Safety Statements | 26-45-36/37/39-27-23 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WK8400000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
STRONTIUM price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 343730 | Strontium random pieces, 99% | 7440-24-6 | 10g | $62.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 343730 | Strontium random pieces, 99% | 7440-24-6 | 50g | $559 | 2023-06-20 | Buy |
| Alfa Aesar | 088098 | Strontium, AAS standard solution | 100mL | $32.65 | 2024-03-01 | Buy | |
| Alfa Aesar | 088098 | Strontium, AAS standard solution | 500mL | $79.65 | 2024-03-01 | Buy | |
| Alfa Aesar | 013874 | Strontium, plasma standard solution | 100mL | $70 | 2024-03-01 | Buy |
STRONTIUM Chemical Properties,Uses,Production
History
William Cruickshank in 1787 and Adair Crawford in 1790 independently detected strontium in the mineral strontianite, small quantities of which are associated with calcium and barium minerals. They determined that the strontianite was an entirely new mineral and was different from baryta and other barium minerals known at the time. In 1808, Sir Humphry Davy isolated strontium by electrolysis of a mixture of moist strontium hydroxide or chloride with mercuric oxide, using a mercury cathode. The element was named after the town Strontian in Scotland where the mineral strontianite was found.
Uses
Strontium is found in small quantities in many rocks and soils, mostly associated with calcium and barium. Its abundance in the earth’s crust is about 370 mg/kg, about the same as barium. The average concentration of this metal in sea water is about 7.9 mg/L.
The two principal strontium minerals are its carbonate, strontianite, SrCO3, and the more abundant sulfate mineral celestite, SrSO4.
Elemental strontium has only minor uses, since most applications involve calcium and barium. Strontium alloys are used as “getters” for vacuum tubes. It is incorporated in glass for making picture tubes for color television. Strontium compounds are used in tracer bullets and in fireworks to produce red signal flares. Strontium titanate is a gemstone. The radioactive strontium- 90 with a half-life of 29 years is a high-energy beta emitter. It is a product of nuclear fission. This isotope is a lightweight nuclear-electric power source in space vehicles and remote weather stations.
Production
Strontium and its compounds are mostly derived from celestite, SrSO4. The mineral is converted to its carbonate by heating with sodium carbonate. Alternatively, the mineral may be reduced to sulfide by heating with coke. The carbonate or the sulfide is then converted to other strontium salts.
Metallic strontium is produced by electrolysis of a mixed melt of strontium chloride and potassium chloride in a graphite crucible using an iron rod as cathode. The upper cathodic space is cooled and the strontium metal collects over the cooled cathode and forms a stick.
Strontium metal also can be prepared by thermal reduction of its oxide with aluminum. Strontium oxide-aluminum mixture is heated at high temperature in vacuum. Strontium is collected by distillation in vacuum. Strontium also is obtained by reduction of its amalgam, hydride, and other salts. The amalgam is heated and the mercury is separated by distillation. If hydride is used, it is heated at 1,000°C in vacuum for decomposition and removal of hydrogen. Such thermal reductions yield high–purity metal.
Hazard
The finely-powdered metal is pyrophoric. Its radioactive isotopes Sr-89 and Sr-90 emit high-energy beta radiation. They are extremely hazardous because they deposit in bones replacing calcium. Their radiation can damage bone marrow and blood-forming organs, inducing cancer.
Description
Strontium has the symbol Sr, the atomic number 38 and an atomic weight of 87.623 g/mol. As an alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. Due to its extreme reactivity with oxygen and water, this element occurs naturally only in compounds with other elements. The metal turns yellow when exposed to air. It occurs naturally in the minerals Celestine(SrSO4) and strontianite(SrCO3). The isotope, 90Sr, is present in radioactive fallout and has a half-life of 28.90 years. The following table presents the abundance of strontium. Strontium commonly occurs in nature, the 15th most abundant element on earth, averaging 0.034% in all igneous rock. It is found chiefly as the form of the sulfate mineral Celestite(SrSO4) and the carbonate Strontianite (SrCO3). Of the two, Celestite occurs much more frequently in sedimentary deposits of sufficient size to make the development of mining facilities attractive. Strontianite is more useful of the two common minerals because strontium is used most often in the carbonate form, but few deposits have been discovered that are suitable for development.
Chemical Properties
Pale-yellow, soft metal; chemically similar to calcium. Soluble in alcohol and acids, decomposes water on contact.
Chemical Properties
Strontium is a silvery-white alkaline-earth metal that rapidly assumes an oxide film and yellow color on exposure to air. Strontium salts impart a brilliant red color to a flame. The finely divided metal ignites spontaneously in air; therefore, the metal should be stored under oxygen-free liquid. Naturally occurring isotopes include 88Sr (82.56%), 86Sr (9.86%), 87Sr, and 84Sr (0.56%). In addition, at least 11 strontium isotopes are produced by fission; of these, the 89Sr and 90Sr isotopes are considered to be environmentally significant. 89Sr emits b-particles with an average energy of 583 keV (1.46 MeV maximum) and has a halflife of 50.5 days. 90Sr is a long-range b-emitter (mean energy 195.8 keV; maximum 540 keV) with a half-life of 28 years. At least 20 strontium salts are known.
Physical properties
In its elemental state, strontium is a relatively soft, pale yellow metal somewhat similar toelemental calcium. When freshly cut, strontium has a silvery shine to its surface that soonturns grayish as it is oxidized by atmospheric oxygen (2Sr + O2 → 2SrO) and nitrogen (3Sr +N2 → Sr3N2), which prevents further oxidation. Strontium’s melting point is 769°C, its boiling point is 1348°C, and its density is 2.54 g/cm3.
Isotopes
Strontium has four naturally occurring isotopes 84Sr
(0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%), but
there are 33 known isotopes (Tables 1.14 and 1.15).
This element (Sr) has four stable, naturally occurring
isotopes: 84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr
(82.58%). Only 87Sr is radiogenic since it is produced
by decay from the radioactive alkali metal 87Rb, which
has a half-life of 4.88×1010 years. Thus, in any material,
there are two sources of 87Sr. That formed during
primordial nucleosynthesis along with 84Sr, 86Sr and
88Sr, and that formed by radioactive decay of 87Rb. The
ratio 87Sr/86Sr is the parameter typically reported in
geologic investigations. The ratios reported in minerals
and rocks have values ranging from 0.7 to greater than
4.0. Because strontium has an electronic configuration
similar to that of calcium, it readily substitutes for Ca
in minerals.
Sixteen unstable isotopes are known to exist. Of greatest
importance are strontium-89 (89Sr) with a half-life of
50.57 days, and strontium-90 (90Sr) with a half-life of
28.78 years. They decay by emitting an electron and an
anti-neutrino (ne) in beta-minus decay (b
decay) to
become yttrium, 90Y (half-life ? 64 h). 89Sr is an artificial
radioisotope that is used in the treatment of bone cancer.
In circumstances where cancer patients have widespread
and painful bony metastases, the administration
of 89Sr results in the delivery of b-particles directly to the
area of the bony problem, where calcium turnover is
greatest. 90Sr is a by-product of nuclear fission found
in “nuclear fallout” and presents a health problem since
it substitutes for calcium in bone, preventing its expulsion
from the body. Significant absorption usually
results in death.
Because it is a long-lived high-energy beta-emitter,
90Sr is used in SNAP (Systems for Nuclear Auxiliary
Power) devices. These devices hold promise for use in
spacecraft, remote weather stations, navigational buoys,
etc., where a lightweight, long-lived, nuclear-electric
power source is required.
Isotopes
There are 29 isotopes of strontium, ranging from Sr-75 to Sr-102. The fournatural forms of strontium are stable and not radioactive. These stable isotopes are Sr-84, which constitutes 0.56% of the element’s existence on Earth; Sr-86, which makesup 9.86%; Sr-87, which accounts for 7.00% of the total; and Sr-88, which makes up82.58% of strontium found on Earth. The remaining isotopes are radioactive with halflives ranging from a few microseconds to minutes, hours, days, or years. Most, but notall, are produced in nuclear reactors or nuclear explosions. Two important radioisotopesare Sr-89 and Sr-90.
Origin of Name
Strontium was named after the town Strontian, located in Scotland in the British Isles.
Occurrence
Strontium metal is not found in its elemental state in nature. Its salts and oxide compoundsconstitute only 0.025% of the Earth’s crust. Strontium is found in Mexico and Spain in the mineral ores of strontianite (SrCO3) and celestite (SrSO4). As these ores are treated with hydrochloricacid (HCl), they produce strontium chloride (SrCl2) that is then used, along with potassiumchloride (KCl), to form a eutectic mixture to reduce the melting point of the SrCl2, as a moltenelectrolyte in a graphite dish-shaped electrolysis apparatus. This process produces Sr cations collected at the cathode, where they acquire electrons to form strontium metal. At the same time,Cl- anions give up electrons at the anode and are released as chlorine gas Cl2↑.Two other methods of producing strontium are by thermal reduction of strontium oxideand by the distillation of strontium in a vacuum.
History
Isolated by Davey by electrolysis in 1808; however, Adair Crawford in 1790 recognized a new mineral (strontianite) as differing from other barium minerals (baryta). Strontium is found chiefly as celestite (SrSO4) and strontianite (SrCO3). Celestite is found in Mexico, Turkey, Iran, Spain, Algeria, and in the U.K. The U.S. has no active celestite mines. The metal can be prepared by electrolysis of the fused chloride mixed with potassium chloride, or is made by reducing strontium oxide with aluminum in a vacuum at a temperature at which strontium distills off. Three allotropic forms of the metal exist, with transition points at 235 and 540°C. Strontium is softer than calcium and decomposes water more vigorously. It does not absorb nitrogen below 380°C. It should be kept under mineral oil to prevent oxidation. Freshly cut strontium has a silvery appearance, but rapidly turns a yellowish color with the formation of the oxide. The finely divided metal ignites spontaneously in air. Volatile strontium salts impart a beautiful crimson color to flames, and these salts are used in pyrotechnics and in the production of flares. Natural strontium is a mixture of four stable isotopes. Thirty-two other unstable isotopes and isomers are known to exist. Of greatest importance is 90Sr with a half-life of 29 years. It is a product of nuclear fallout and presents a health problem. This isotope is one of the best long-lived high-energy beta emitters known, and is used in SNAP (Systems for Nuclear Auxiliary Power) devices. These devices hold promise for use in space vehicles, remote weather stations, navigational buoys, etc., where a lightweight, long-lived, nuclear-electric power source is needed. The major use for strontium at present is in producing glass for color television picture tubes. All color TV and cathode ray tubes sold in the U.S. are required by law to contain strontium in the face plate glass to block X-ray emission. Strontium also improves the brilliance of the glass and the quality of the picture. It has also found use in producing ferrite magnets and in refining zinc. Strontium titanate is an interesting optical material as it has an extremely high refractive index and an optical dispersion greater than that of diamond. It has been used as a gemstone, but it is very soft. It does not occur naturally. Strontium metal (99% pure) costs about $220/kg.
Characteristics
When strontium metal is exposed to water, it releases hydrogen, as do the other earth metals (Sr + 2H2O → Sr(OH)2 + H2↑). Strontium can ignite when heated above its melting point.When in a fine powder form, it will burn spontaneously in air. It must be stored in an inertatmosphere or in naphtha. Several of its salts burn with a bright red flame, making it usefulin signal flares and fireworks.
History
Both strontium and Strontianite are named after Strontian, a village in Scotland near which the mineral was first discovered in the ores taken from the lead mines. In 1787, an intriguing mineral came to Edinburgh from a Lead mine in a small village on the shores of Loch Sunart, Argyll, in the western highlands of Scotland. At that time, the substance was thought to be some sort of Barium compound. It was 3 years later that Scott s Irish chemist, Adair Crawford, published a paper claiming that the mineral held a new species including a new chemical element. Other chemists later prepared a number of compounds with the element, noting that it caused the candle s flame to burn red, while barium compounds gave a green color. The new mineral was named Strontite in 1793 by Thomas Hope, another professor of medicine at the University of Glasgow. This element was eventually isolated by Humphrey Davy in 1808 during his studies of the electrolysis of various alkaline earths containing molten chloride such as SrCl2 and mercuric oxide. He announced his work in a lecture to the Royal Society on 30 June 1808. In keeping with the naming of the other alkaline earths, he changed the name to Strontium.
Uses
In fireworks, in red signal flares; on tracer bullets. Eutectic modifier in Al-Ag casting alloys to improve strength and ductility. Innoculant in ductile iron casting to control graphite formation.
Uses
Strontium does not have as many practical uses as do some of the other alkali earth metals.Strontium nitrate [Sr(NO3)2], when burned, produces a bright red flame, and it is usedin fireworks. During military combat, it is used to make “tracer bullets” so that their pathscan be tracked at night. Strontium is also used in making specialty metals when alloyed withother metals and in the manufacture of soaps, greases, and similar materials that are resistantto extreme high or low temperatures.
Uses
This soft, yellowish, metallic element was isolated by Sir Humphry Davy in 1808. It was found in the minerals strontianite and celestine. The strontium halides were used in the making of collodion emulsions.
Definition
Metallic element of atomic number 38, group IIA of periodic table, aw 87.62, valence = 2, radioactive isotopes strontium-89 and strontium-90. There are four stable isotopes.
Definition
strontium: Symbol Sr. A soft yellowish metallic element belonging to group 2 (formerly IIA) of the periodic table (see alkaline-earth metals); a.n. 38; r.a.m. 87.62; r.d. 2.6; m.p. 769°C; b.p. 1384°C. The element is found in the minerals strontianite (SrCO3) and celestine (SrSO4). It can be obtained by roasting the ore to give the oxide, followed by reduction with aluminium (i.e. the Goldschmidt process). The element, which is highly reactive, is used in certain alloys and as a vacuum getter. The isotope strontium–90 is present in radioactive fallout (half-life 28 years), and can be metabolized with calcium so that it collects in bone. Strontium was discovered by Martin Klaproth (1743–1817) and Thomas Hope (1766–1844) in 1798 and isolated by Humphry Davy in 1808.
Definition
A soft low-melting reactive metal; the fourth member of group 2 of the periodic table and a typical alkaline-earth element. The electronic configuration is that of krypton with two additional outer 5s electrons. Strontium and barium are both of low abundance in the Earth’s crust, strontium occurring as strontianite (SrCO3) and celestine (SrSO4).
The element is produced industrially by roasting the carbonate to give the oxide (800°C) and then reducing with aluminum, 3SrO + 2Al → Al2O3 + 2Sr
Strontium has a low ionization potential, is large, and is therefore very electropositive. The chemistry of strontium metal is therefore characterized by high reactivity of the metal. The properties of strontium fall into sequence with other alkaline earths. Thus it reacts directly with oxygen, nitrogen, sulfur, the halogens, and hydrogen to form respectively the oxide SrO, nitride Sr3N2, sulfide SrS, halides SrX2, and hydride SrH2, all of which are largely ionic in character. The oxide SrO and the metal react readily with water to form the hydroxide Sr(OH)2, which is basic and mid-way between Ca(OH)2 and Ba(OH)2 in solubility. The carbonate and sulfate are both insoluble.
As the metal is very electropositive the salts are never much hydrolyzed in solution and the ions are largely solvated as [Sr(H2O)6]2+. Symbol: Sr; m.p. 769°C; b.p. 1384°C; r.d. 2.54 (20°C); p.n. 38; r.a.m. 87.62.
Hazard
Spontaneously flammable in powder form, igniteswhen heated above its mp. Reactswithwater to evolve hydrogen. Store under naphtha.
Hazard
As a powder, strontium metal may spontaneously burst into flames. Both its metal andsome of its compounds will explode when heated. Some of the compounds will explode ifstruck with a hammer.
Both the metal and some compounds will react with water to produce strontium hydroxide[Sr(OH)2] and release hydrogen gas. The heat from the exothermic reaction may cause thehydrogen to either burn or explode [Sr + 2H2O → Sr(OH)2 + H2↑].
Some compounds, such as strontium chromate and strontium fluoride, are carcinogensand toxic if ingested. Strontium-90 is particularly dangerous because it is a radioactivebone-seeker that replaces the calcium in bone tissue. Radiation poisoning and death mayoccur in people exposed to excessive doses of Sr-90. Strontium-90, as well as some otherradioisotopes that are produced by explosions of nuclear weapons and then transportedatmospherically, may be inhaled by plants and animals many miles from the source of thedetonation. This and other factors led to the ban on atmospheric testing of nuclear andthermonuclear weapons.
Flammability and Explosibility
Substances and mixtures which in contact with water emit flammable gases
Carcinogenicity
The carcinogenicity of stable (nonradioactive) strontium chromate was attributed solely to intracellular soluble chromium. 90Sr has been examined in long-term studies in four species, involving beagles, mice, monkeys, and pigs. A summary of the findings of these studies can be found in Ref.. Following intravenous injection of 90Sr at doses ranging from 0.027 to 362×104 Bq/kg, the most prominent 90Sr -induced endpoint was bone sarcoma. Neoplasms involving the soft tissues near bone in the oronasopharynx and paranasal sinuses and bone marrow dysplasia were also significantly elevated over controls. Feeding studies in beagles extending from the in utero period to age 540 days resulted in the development of the same array of tumors, and, in addition, myeloproliferative disorders. Inhalation exposure to 90Sr Cl2 was associated with multiple carcinogenic and non-neoplastic lesions in dogs, with an excess of bone tumors reported as the major finding. Interestingly, inhalation exposure of dogs to insoluble forms of 90Sr was associated with lung tumors, but not bone tumors. In an additional study in which beagle dogs were injected with lowlevels of 90Sr (21.1 kBq/kg, or five times the maximum permissible (retained) body burden), 90Sr was not associated with a decrease in survival time in dogs. It has been estimated that 90Sr is approximately two orders of magnitude less toxic than radium. Two monkey studies were also summarized by the Council on Radiation Protection and Measurements. One of these studies involved administration of single intravenous injections of 90Sr (0.10–6.21 MBq) to rhesus monkeys. These monkeys had no symptoms or disease attributable to 90Sr 20 years after exposure. In another study, administration of 1.85 or 3.7 MBq of 90Sr as a single oral dose resulted in bone sarcomas.
Environmental Fate
Most stable strontium and some radioactive strontium compounds exist as dust in air, which eventually settles over land and water. Stable strontium dissolves in water and moves deeper in soil to underground water. The solid is found suspended in water. Strontium is also found naturally in soil due to the release of coal ash and industrial wastes. Soluble strontium compounds, through chemical reactions, can transform to insoluble and vice versa. The long half-life of strontium-90 (29 years) can allow airborne particles to move all around the world.
Toxicity evaluation
Strontium’s inherent toxicity and that of its compounds resembles that of calcium. The state of calcium nutrition of exposed individuals is a major determinant of toxicity. The radioactive isotope, when ingested or inhaled, is processed by the body and resides in bones. Strontium ionizes molecules in the body by the emission of beta particles. It increases the risk of cancer.
STRONTIUM Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 8032 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | sales@senfeida.com | China | 23048 | 58 |
| Changzhou AniKare Pharmatech Co., Ltd. | +86-0519-8359-8696 +8618018249389 | sales@anikare.com | China | 9259 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
Related articles
- Strontium: Major Minerals, Chemistry properties and Reactions
- Strontium (Sr) is a soft silver-white yellowish metallic element. It is a member of alkaline earth elements (Group 2) and occu....
- May 29,2024
- How funny strontium flame test
- Positive result of a flame test for strontium (Sr), producing a red colour. This is due to the excitation of electrons in the ....
- Jan 24,2024
- Strontium Industrial Applications and Uses
- Strontium chemical symbol Sr, atomic number 38, and relative atomic mass (i.e., atomic weight) 87.62, is the fourth alkaline-e....
- Sep 25,2019
7440-24-6(STRONTIUM)Related Search:
1of4