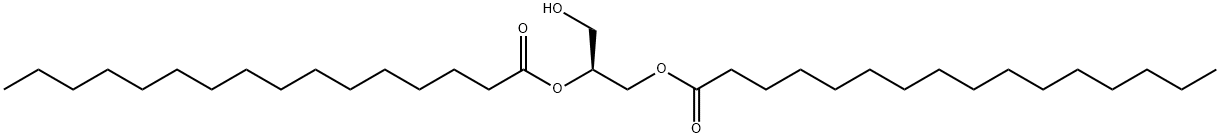
1,2-DIPALMITOYL-SN-GLYCEROL
- CAS No.
- 30334-71-5
- Chemical Name:
- 1,2-DIPALMITOYL-SN-GLYCEROL
- Synonyms
- DPG;(S)-3-Hydroxypropane-1,2-diyl dipalmitate;1,2-DIPALMITIN;DG(16:0/16:0/0:0);16:0 DG;NSC 269964;Einecs 250-131-4;D-a,b-DipalMitin;sn-1,2-DipalMitin;(S)-1,2-DIPALMITIN
- CBNumber:
- CB4228240
- Molecular Formula:
- C35H68O5
- Molecular Weight:
- 568.91
- MDL Number:
- MFCD00067471
- MOL File:
- 30334-71-5.mol
- MSDS File:
- SDS
| Melting point | 66-69 °C |
|---|---|
| Boiling point | 620.8±22.0 °C(Predicted) |
| Density | 0.930±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMF: 20 mg/ml; DMSO: 5 mg/ml; Ethanol: 30 mg/ml; PBS (pH 7.2): .25 mg/ml |
| form | A crystalline solid |
| pka | 13.69±0.10(Predicted) |
| BRN | 1730209 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H315-H319-H331-H336-H351-H361d-H372-H412 |
| Precautionary statements | P201-P273-P301+P312+P330-P302+P352-P304+P340+P311-P308+P313 |
| WGK Germany | 1 |
| F | 10 |
1,2-DIPALMITOYL-SN-GLYCEROL price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D9135 | 1,2-Dipalmitoyl-sn-glycerol ≥99% | 30334-71-5 | 25mg | $49.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | D9135 | 1,2-Dipalmitoyl-sn-glycerol ≥99% | 30334-71-5 | 100mg | $78.6 | 2024-03-01 | Buy |
| Cayman Chemical | 10008648 | 1,2-Dipalmitoyl-sn-glycerol ≥95% | 30334-71-5 | 25mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 10008648 | 1,2-Dipalmitoyl-sn-glycerol ≥95% | 30334-71-5 | 50mg | $55 | 2024-03-01 | Buy |
| Cayman Chemical | 10008648 | 1,2-Dipalmitoyl-sn-glycerol ≥95% | 30334-71-5 | 100mg | $81 | 2024-03-01 | Buy |
1,2-DIPALMITOYL-SN-GLYCEROL Chemical Properties,Uses,Production
Uses
1,2-Dipalmitoyl-sn-glycerol (1,2-DPG) is an analog of the protein kinase C (PKC)-activating second messenger diacylglycerol. 1,2-Dipalmitoyl-sn-glycerol is a weak activator of PKC.
Uses
16:0 DG has been used to spike brain samples for mass spectrometric analysis. It may be used as diacyl-glycerol source in diacylglycerol O-acyltransferase 1 (DGAT1) enzyme assay in human leukemic?K562 cells and in Golgi-like liposomes reconstitution.
Definition
ChEBI: A 1,2-diacyl-sn-glycerol in which both acyl groups are specified as palmitoyl (hexadecanoyl).
General Description
In biochemical signaling, diacylglycerol (DAG) functions as a second messenger signaling lipid, and is a product of the hydrolysis of the phospholipid PIP2 (phosphatidylinositolbisphosphate) by the enzyme phospholipase C (PLC) (a membrane-bound enzyme) that, through the same reaction, produces inositol trisphosphate (IP3). Although inositol trisphosphate (IP3) diffuses into the cytosol, DAG remains within the plasma membrane due to its hydrophobic properties. IP3 stimulates the release of calcium ions from the smooth endoplasmic reticulum, whereas DAG is a physiological activator of protein kinase C (PKC). The production of DAG in the membrane facilitates translocation of PKC from the cytosol to the plasma membrane.
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Biochem/physiol Actions
16:0 DG (1,2-dipalmitoyl-sn-glycerol) fails to prevent the late fission events in Golgi membrane. Supplementation of 16:0 DG promotes bacterial growth by preventing sporangia.
Purification Methods
Crystallise S(-)-1,2-dipalmitin from chloroform/pet ether (b 40-60o) ~1:1.5. [S(-)-isomer: Baer & Kates J Am Chem Soc 72 942 1950, Hanahan & Vercamer J Am Chem Soc 7 6 1804 1954, R(+)-isomer: Tattrie et al. Arch Biochem 78 319 1958, Beilstein 2 IV 1173.] The racemate [40290-32-2] is polymorphic with different IR spectra. When crystallised from hexane, or other solvents, the higher melting form with m 71.5-72.5o is obtained. The melt then solidifies to give the lower melting -form with m 49.7-50o. The -form has m 61o (65-66o is also reported). When the lower melting forms are kept at their melting temperatures for a while, they are converted to the higher melting form. [Howe & Malkin J Chem Soc 2663 1951, Baer & Kates J Am Chem Soc 72 942 1950, Beilstein 2 IV 1173.]
1,2-DIPALMITOYL-SN-GLYCEROL Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Shanghai Sinch Parmaceuticals Tech. Co. Ltd. | +86-21-54098501 | sales@sinch.com.cn | China | 11605 | 64 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Tianjin Derchemist Science & Technology Co., Ltd. | 22-58627059 13920586291 | zdcomcon@126.com | China | 2918 | 50 |
30334-71-5(1,2-DIPALMITOYL-SN-GLYCEROL)Related Search:
1of4