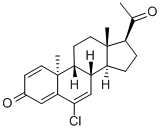
trengestone
- CAS No.
- 5192-84-7
- Chemical Name:
- trengestone
- Synonyms
- trengestone;(9β,10α)-6-Chloropregna-1,4,6-trien-3,20-dione;(9β,10α)-6-Chloropregna-1,4,6-triene-3,20-dione;Pregna-1,4,6-triene-3,20-dione, 6-chloro-, (9β,10α)-;(9R,10S,13S,17S)-17-acetyl-6-chloro-10,13-dimethyl-8,9,11,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one;(9R,10S,13S,17S)-6-chloro-17-ethanoyl-10,13-dimethyl-8,9,11,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
- CBNumber:
- CB4893578
- Molecular Formula:
- C21H25ClO2
- Molecular Weight:
- 344.88
- MDL Number:
- MOL File:
- 5192-84-7.mol
| Melting point | 208-209° (dec) |
|---|---|
| Boiling point | 478.6±45.0 °C(Predicted) |
| Density | 1.20±0.1 g/cm3(Predicted) |
| FDA UNII | VY6S496SVX |
trengestone Chemical Properties,Uses,Production
Originator
Retrone,Roche
Manufacturing Process
10 g of 9β,10α-pregna-4,6-diene-3,20-dione (Rec. Trav. Chim. 79, 771
(1960)) were dissolved in a solution of 26 g of perbenzoic acid in 565 ml of
chloroform at 0°C, after which the reaction mixture was allowed to stand at
the same temperature in a refrigerator. The consumption of peracid was
determined by iodometrical titration, where by the decrease of content of
peracid of a blank was taken into account. After 1, 5, 20, 29 and 45 hours 18,
55, 155, 173 and 190% of peracid were consumed, respectively. After a reaction time of 20, 27 and 46 hours samples of the reaction mixture
contained according to the ultraviolet absorption spectrum 24, 18 and 16.4%
of the starting material, respectively. After 50 hours the reaction mixture was
diluted with 2 1 of ether and washed (at 0°C) with 4 x 750 ml of a 3 %
sodium carbonate solution and then with 3 x 500 ml of ice-water (until
neutral). The solution was dried over sodium sulphate, filtered and the solvent
evaporated in vacuo at 30°C. The resin (9.02 g), showed a maximum at 234
and 285 nm.
A small sample of the epoxydation product was purified chromatographically
(silicage) for analytical purposes. Melting point: 186(s)-189°C, ε (λmax 241
nm) = 14000. The infrared absorption spectrum showed bands at 1703, 1677,
1627, 1354, 1233, 953, 881 and 808 cm-1. The crude epoxidation product
was dissolved in 400 ml of ethanol-free chloroform. To this solution were
added 280 ml of a 6.5% solution of hydrochloric acid in acetic acid, after
which the dark colored reaction mixture was allowed to stand at room
temperature for 3.5 hours. Then the reaction mixture was poured into 2 1 of
ice-water and the water layer extracted once with chloroform. The combined
chloroform layers were washed with 3 x 1500 ml of ice-water, 3 x 500 ml of
5% sodium bicarbonate solution at 0°C and finally with water until neutral
After drying over sodium sulphate the solution was filtered and the solvent
evaporated in vacuo. The resinous residue was treated with a small amount of
cold ether by which 1.5 g of crystals were obtained. Recrystallization from
ethyl acetate at -5°C yielded 0.76 g of crystals with a melting point of 168-
170°C (decomposition), λmax 287 nm. Repeated recrystallization finally gave
0.64 g of 6-chloro-9β,10α-pregna-4,6-diene-3,20-dione with a melting point of
169(s)-175.5°C (dec.). The melting (decomposition) point of the substance
proved to be no standard for the purity of the compound. ε (λmax = 21600).
A solution of 1.04 g of 6-chloro-9β,10α-pregna-4,6-diene-3,20-dione and 0.95
g of 2,3-dichlorobenzoquinone in 50 ml of dry benzene was heated to reflux
for 10 hours. The reaction mixture was diluted with 70 ml of benzene and
extracted three times with 50 ml of 2 N sodium hydroxide solution. The
benzene layer was washed with water to neutral, dried with sodium sulfate
and evaporated to dryness. The residue (0.7 g) was chromatographed on 20 g
0f aluminum oxide (activity II). The fraction eluted with benzene-petroleum
ether were combined and recrystallized from acetone. The 6-chloro-9β,10α-
pregna-1,4,6-triene-3,20-dione melted at 208-209°C(decomposition), λmax
229 nm.
Therapeutic Function
Progestin
trengestone Preparation Products And Raw materials
Raw materials
Preparation Products
trengestone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10008 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 58 |