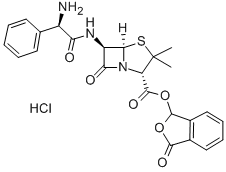
Talampicillin hydrochloride
- CAS No.
- 39878-70-1
- Chemical Name:
- Talampicillin hydrochloride
- Synonyms
- Talat;PC 183;Talpen;BRL 8988;Yamacillin;Aseocillin;Ampicillin Impurity 26;D-Talampicillin hydrochloride;Talampicillin hydrochloride CRS;Ampicillinphthalidyl hydrochloride
- CBNumber:
- CB51105387
- Molecular Formula:
- C24H23N3O6S.ClH
- Molecular Weight:
- 517.988
- MDL Number:
- MFCD01682149
- MOL File:
- 39878-70-1.mol
| Melting point | 154-157° (dec) |
|---|---|
| FDA UNII | 4C5O3X072I |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H317-H319-H334-H335 |
| Precautionary statements | P280-P302+P352-P305+P351+P338 |
Talampicillin hydrochloride price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T0010000 | Talampicillin hydrochloride European Pharmacopoeia (EP) Reference Standard | 39878-70-1 | t0010000 | $150 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | API0016125 | TALAMPICILLIN HYDROCHLORIDE 95.00% | 39878-70-1 | 10MG | $280.35 | 2021-12-16 | Buy |
Talampicillin hydrochloride Chemical Properties,Uses,Production
Description
Talampicillin was synthesized by Yamanouchi Pharmaceutical Co. in 1971 by esterifying the carboxylic acid group of ampicillin in order to improve the oral absorption. When administered orally, it is hydrolyzed to ampicillin by intestinal esterase. Its bioavailability is two or more times as high as that of ampicillin, and it is used to treat the same infections as those for which ampicillin is used orally but in doses only half or one-third as large.
Originator
Talpen,Beecham,US,1975
Manufacturing Process
A fine suspension of 25.18 grams (0.05 mol) of potassium salt of enamine protected ampicillin and 10.65 grams (0.05 mol) 3-bromophthalide were reacted in a 1:2 mixture of acetone/ethyl acetate (1,500 ml) for 24 hours. After filtration the organic layer was washed twice with 250 ml portions of 1 N sodium bicarbonate and brine, dried over anhydrous magnesium sulfate and concentrated in vacuo. Addition of ether crystallized the phthalide enamine protected α-aminophenylacetamido penicillanate in 85% yield.
Therapeutic Function
Antibacterial
Talampicillin hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Alta Scientific Co., Ltd. | 022-6537-8550 15522853686 | sales@altasci.com.cn | China | 4515 | 55 |
| Hubei Moco Chemical Co., Ltd. | 18627756402 | 3001051413@qq.com | China | 10009 | 58 |
| Nanjing wangzhixing Pharmaceutical Technology Co., Ltd | 15850510341 13101896326 | shiqingran@wzxchem.com | China | 1967 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 0755-66853366 13670046396 | orders@qcsrm.com | China | 18810 | 58 |
39878-70-1(Talampicillin hydrochloride)Related Search:
1of4