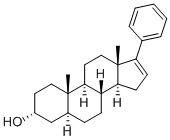
17-PA
- CAS No.
- 694438-95-4
- Chemical Name:
- 17-PA
- Synonyms
- 17-PA;17PA,17 PA;17-Phenyl-(3α,5α)-androst-16-en-3-ol;17-PHENYL-(3A,5A)-ANDROST-16-EN-3-OL;17-Phenyl-(3α,5α)-androst-16-en-3-ol;Androst-16-en-3-ol, 17-phenyl-, (3α,5α)-
- CBNumber:
- CB52453436
- Molecular Formula:
- C25H34O
- Molecular Weight:
- 350.54
- MDL Number:
- MFCD09971119
- MOL File:
- 694438-95-4.mol
17-PA price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Tocris | 2681 | 17-PA ≥99%(HPLC) | 694438-95-4 | 10 | $217 | 2021-12-16 | Buy |
| Tocris | 2681 | 17-PA ≥99%(HPLC) | 694438-95-4 | 50 | $870 | 2021-12-16 | Buy |
| Usbiological | 254348 | 17-PA | 694438-95-4 | 10mg | $579 | 2021-12-16 | Buy |
| TRC | P133880 | 17-PA | 694438-95-4 | 25mg | $365 | 2021-12-16 | Buy |
| ApexBio Technology | B7151 | 17-PA | 694438-95-4 | 10mg | $408 | 2021-12-16 | Buy |
17-PA Chemical Properties,Uses,Production
Uses
17-PA is an antagonist of neurosteroid GABA potentiation.
Biological Activity
Selective antagonist of neurosteroid potentiation and direct gating of GABA A receptors. Selectively reduces the effects of 5 α -reduced steroids compared to 5 β -reduced steroids and displays no effect on potentiation evoked by barbiturates and benzodiazepines. Attenuates 3 α ,5 α -THP-induced loss of righting reflex and total sleep time following i.c.v administration in rats.
Enzyme inhibitor
This selective neurosteroid antagonist (FW = 350.54 g/mol; CAS 694438- 95-4; Soluble to 25 mM in DMSO and to 50 mM in ethanol), also known as 17-PA, targets neurosteroid potentiation and directly gates GABAA receptors. 17-PA inhibition was also useful in demonstrating that ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the α1β2γ2L GABAA receptor. 17-PA selectively reduces the effects of 5α-reduced steroids compared to 5β-reduced steroids and displays no effect on potentiation evoked by barbiturates and benzodiazepines. It also attenuates 3α,5α-THP-induced loss of righting reflex and total sleep time, following intracerebroventricular administration in rats.
17-PA Preparation Products And Raw materials
Raw materials
Preparation Products
17-PA Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8449 | 60 |
| Shanghai Rechem science Co., Ltd. | 21-31433387 15618786686 | sales@rechemscience.com | China | 2983 | 58 |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |
| MedBioPharmaceutical Technology Inc | 021-69568360 18916172912 | order@med-bio.cn | China | 8141 | 58 |
| JinOu Biomedical (Nanjing) Co., Ltd. | 13000000000 | jinoupharma@163.com | China | 11732 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Shanghai Tachizaki Biomedical Research Center | 18014399201 | sales@chemlab-tachizaki.com | China | 2488 | 58 |