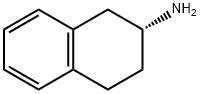
(R)-2-AMINOTETRALIN
- CAS No.
- 21966-60-9
- Chemical Name:
- (R)-2-AMINOTETRALIN
- Synonyms
- (R)-2-AMINOTETRALIN;(2R)-Tetralin-2α-amine;(R)-(+)-2-AMINOTETRALIN;(R)-1,2,3,4-Tetrahydro-2-naphthylam;(R)-1,2,3,4-Tetrahydro-2-naphthylamine;(R)-1,2,3,4-Tetrahydronaphthalen-2-amine;(2R)-1,2,3,4-tetrahydronaphthalen-2-amine;(R)-1,2,3,4-Tetrahydronaphthalene-2-amine;(R)-1,2,3,4-Tetrahydro-naphthalen-2-ylaMine;2-Naphthalenamine, 1,2,3,4-tetrahydro-, (2R)-
- CBNumber:
- CB5249280
- Molecular Formula:
- C10H13N
- Molecular Weight:
- 147.22
- MDL Number:
- MFCD00671631
- MOL File:
- 21966-60-9.mol
- MSDS File:
- SDS
SAFETY
Risk and Safety Statements
| Risk Statements | 36/37/38 |
|---|---|
| Safety Statements | 26-36/37/39 |
(R)-2-AMINOTETRALIN price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | T796403 | (R)-1,2,3,4-Tetrahydronaphthalen-2-amine | 21966-60-9 | 50mg | $175 | 2021-12-16 | Buy |
| AK Scientific | 1181AL | (R)-1,2,3,4-Tetrahydro-2-naphthylamine | 21966-60-9 | 5g | $2176 | 2021-12-16 | Buy |
| Matrix Scientific | 131974 | (R)-1,2,3,4-Tetrahydronaphthalen-2-amine 97% | 21966-60-9 | 5g | $2340 | 2021-12-16 | Buy |
| Matrix Scientific | 131974 | (R)-1,2,3,4-Tetrahydronaphthalen-2-amine 97% | 21966-60-9 | 10g | $4212 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CCH0001190 | (R)-1,2,3,4-TETRAHYDRO-2-NAPHTHYLAMINE 95.00% | 21966-60-9 | 0.25G | $271 | 2021-12-16 | Buy |
(R)-2-AMINOTETRALIN Chemical Properties,Uses,Production
Synthesis
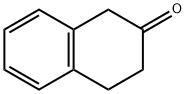
530-93-8
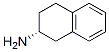
2217-42-7

2217-42-7
The general procedure for the synthesis of the target compound (CAS:2217-42-7) using β-tetralone as starting material was as follows: the final concentration of the reaction system was 0.25 μl, 100 mM potassium phosphate (KPi) buffer at pH 7.5 was used, and an equimolar amount of 70 mM of amino donor was added to 70 mM of ketone substrate in 0.1 ml of cell free extract (CFE) containing transaminase ) was reacted at 28°C for 24 hours. The specific reaction conditions were as follows: the CFE containing transaminases XP-001209325, AAN21261 and ABN35871 were co-incubated with benzylacetone and α-methylbenzylamine to produce 4-phenyl-2-butylamine and acetophenone; with phenylacetone and α-methylbenzylamine to produce α-ethylbenzylamine and acetophenone; and with 1-indanone and α-methylbenzylamine to produce 1-aminoindan and acetophenone; co-incubation with 1-tetralone and α-methylbenzylamine to form 1-aminotetralone and acetophenone; co-incubation with 2-tetralone and α-methylbenzylamine to form 2-aminotetralone and acetophenone; co-incubation with butanone and α-methylbenzylamine to form 2-aminobutane and acetophenone; co-incubation with 3,3-dimethyl-2-butanone and α-methylbenzylamine to form, respectively 3,3-dimethyl-2-aminobutane and acetophenone, respectively. Reaction termination was achieved by adding 0.75 ml of termination reagent (50% (v/v) aqueous acetonitrile solution containing 0.1% (v/v) formic acid) to 0.25 ml of the reaction system. The product concentration and enantiomeric excess values were determined by HPLC analysis, and the results are detailed in Table 6.In addition, CFE containing transaminase YP-955297 was co-incubated with acetophenone and α-methylbenzylamine, which similarly yielded α-ethylbenzylamine and acetophenone, and the reaction was terminated and analyzed as above. After incubation at 28°C for 24 h, 0.87 mmol/l of (R)-α-ethylbenzylamine was obtained with a high enantiomeric excess value. The above results confirmed that transaminases XP-001209325 and YP-955297 are highly selective (R)-transaminases. Further studies showed that the substrate specificity of transaminase XP-001209325 differed from that of AAN21261 and ABN35871: XP-001209325 was able to catalyze significantly the generation of 4-phenyl-2-butylamine, whereas AAN21261 and ABN35871 did not (Table 6). In addition, XP-001209325 catalyzed the generation of enantiomerically enriched (R)-2-aminobutane from 2-butanone, whereas ABN35871 predominantly generated (S)-2-aminobutane (Table 6).
References
[1] ACS Catalysis, 2013, vol. 3, # 4, p. 555 - 559
[2] ACS Catalysis, 2013, vol. 3, # 4, p. 555 - 559
[3] Patent: US2016/32335, 2016, A1. Location in patent: Paragraph 0120; 0121; 0122
(R)-2-AMINOTETRALIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49733 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29804 | 58 |
| SIMAGCHEM CORP | +86-5922680277 +86-13806087780 | sale@simagchem.com | China | 17346 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Win-Win chemical CO., Limited | +86-0086-577-64498589 +86-15355981851 | sales@win-winchemical.com | China | 14459 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9414 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | info@dycnchem.com | China | 52846 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49734 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49983 | 58 |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 | ellena@amitychem.com | China | 43416 | 58 |
View Lastest Price from (R)-2-AMINOTETRALIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-01-13 | (R)-2-AMINOTETRALIN
21966-60-9
|
US $1.00 / KG | 1KG | 98% | 10 tons | Career Henan Chemical Co |
-

- (R)-2-AMINOTETRALIN
21966-60-9
- US $1.00 / KG
- 98%
- Career Henan Chemical Co
21966-60-9((R)-2-AMINOTETRALIN)Related Search:
1of4