ChemicalBook >> CAS DataBase List >>Cabozantinib impurity DX2
Cabozantinib impurity DX2
- CAS No.
- 1628530-38-0
- Chemical Name:
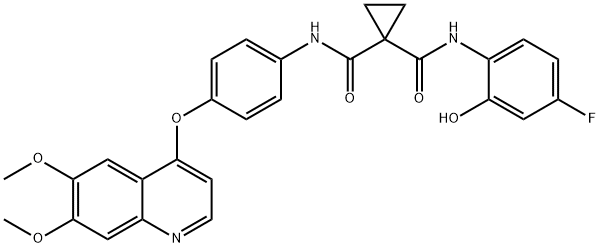
- Cabozantinib impurity DX2
- Synonyms
- Cabozantinib Impurity 78;Cabozantinib impurity 65;Cabozantinib impurity DX2;Cabozantinib Metabolite M9;Tributyl Acetylcitrate Impurity 1 (Tributyl Acetylcitrate EP Impurity A);N-(4-((6,7-Dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluoro-2-hydroxyphenyl)cyclopropane-1,1-dicarboxamide;N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluoro-2-hydroxyphenyl)-1,1-cyclopropanedicarboxamide;1,1-Cyclopropanedicarboxamide, N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluoro-2-hydroxyphenyl)-;N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluoro-2-hydroxyphenyl)cyclopropane-1,1-dicarboxamide? (Cabozantinib Impurity)
- CBNumber:
- CB55590037
- Molecular Formula:
- C28H24FN3O6
- Molecular Weight:
- 517.51
- MDL Number:
- MOL File:
- 1628530-38-0.mol
Last updated:2023-05-15 10:43:29
| Boiling point | 781.8±60.0 °C(Predicted) |
|---|---|
| Density | 1.447±0.06 g/cm3(Predicted) |
| pka | 8.42±0.40(Predicted) |
| FDA UNII | XU28OQ19RG |
Cabozantinib impurity DX2 Preparation Products And Raw materials
Raw materials
Preparation Products
Cabozantinib impurity DX2 Suppliers
Global( 15)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 | greensnown@163.com | China | 9911 | 50 |
| Hubei Yangxin Medical Technology Co., Ltd. | 15374522761 | 3003392093@qq.com | China | 7851 | 55 |
| Zhaoqing Wanwei New Material Technology Co., Ltd. | 0755-1328880-4488 13068786899 | 13288804488@163.com | China | 625 | 58 |
| Shenzhen ruikai de biotechnology co., ltd. | 0755-61348847 17820674378 | 2682116078@qq.com | China | 7844 | 58 |
| Wuhan chuanghao Biotechnology Co., Ltd | 13361917963 | 876725023@qq.com | China | 297 | 58 |
| Shenzhen Hengfeng Wanda Pharmaceutical Technology Co., Ltd | 755-22677922-801 18126518403 | 2851922760@qq.com | China | 5043 | 58 |
| QUALITY CONTROL SOLUTIONS LTD. | 13670046396 | ORDERS@QCSRM.COM | China | 18810 | 58 |
| Nanjing Shanyao Kangcheng biomedical Co., Ltd. | 15722982350 | 1170703875@qq.com | China | 516 | 58 |
| Bide Pharmatech Ltd. | 400-1647117 15221909166 | product02@bidepharm.com | China | 61720 | 58 |
| Bide Pharmatech Ltd. | 400-6005915 | sales@picasso-e.com | China | 39668 | 58 |
1628530-38-0(Cabozantinib impurity DX2)Related Search:
4-Aminoveratrole
Cabozantinib Impurity 5
N-(4-fluorophenyl)-N-(4-hydroxyphenyl)cyclopropane-1,1-dicarboxamide
4-chloro-6,7-dimethoxyquinoline
Cabozantinib Impurity EXA
1-(4-Fluorophenylcarbamoyl)cyclopropanecarboxylic acid
Cyclopropanecarboxylic acid, 1-[[[4-[[7-methoxy-6-(sulfooxy)-4-quinolinyl]oxy]phenyl]amino]carbonyl]-
1,1-Cyclopropanedicarboxamide,N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-phenyl-
Quinoline, 6,7-dimethoxy-4-(4-nitrophenoxy)-
Cyclopropanecarboxylic acid, 1-[(phenylaMino)carbonyl]-
1of4
N-[4-[(6,7-Dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluoro-2-hydroxyphenyl)-1,1-cyclopropanedicarboxamide
Cabozantinib impurity DX2
Cabozantinib impurity 65
1,1-Cyclopropanedicarboxamide, N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-(4-fluoro-2-hydroxyphenyl)-
N-(4-((6,7-Dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluoro-2-hydroxyphenyl)cyclopropane-1,1-dicarboxamide
Cabozantinib Metabolite M9
Cabozantinib Impurity 78
Tributyl Acetylcitrate Impurity 1 (Tributyl Acetylcitrate EP Impurity A)
N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluoro-2-hydroxyphenyl)cyclopropane-1,1-dicarboxamide? (Cabozantinib Impurity)
1628530-38-0