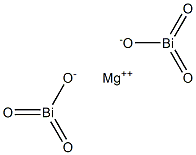
Magnesium bismuthate
- CAS No.
- Chemical Name:
- Magnesium bismuthate
- Synonyms
- Magnesium bismuthate
- CBNumber:
- CB74865978
- Molecular Formula:
- Bi2MgO6
- Molecular Weight:
- 538.26216
- MDL Number:
- MOL File:
- Mol file
Magnesium bismuthate Chemical Properties,Uses,Production
Description
This compound is semiconducting and is
employed in small batteries used in portable electronic
devices. Magnesium bismuthate is prepared by the reaction
of sodium bismuthate with a soluble Mg salt.
MgBi2O6 can be classified as a transparent conducting
oxide (TCO) that possess an unusual combination
of properties; high transparency across most of the
visible spectrum, coupled with high-electrical conductivity.
TCO’s have a wide variety of applications,
including use in flat panel displays, solar cells,
energy-efficient windows, gas sensors, antistatic coatings,
and heating elements.
Physical properties
MgBi2O6 reveals temperature-
independent conductivity (0.01 S/cm2),
a negative Seebeck coefficient (20.025 mV/K), and an
optical band gap that falls at the low-energy end of
visible region (1.8 eV). This combination of attributes,
indicating that this compound is a degenerate n-type
semiconductor, a trait that has not previously been
reported in a Bi5+ oxide.
It consists of a ring structure of octahedral BiO62-
octagonal units with interspersed Mg2+ cations. Characterization
of polycrystalline samples of the trirutile
oxide Bi5+ revealed temperature-independent
conductivity (0.4 and 0.01 S/cm), a negative Seebeck
coefficient ( 0.035 and 0.025 mV/K), and an optical
band gap that falls at the low-energy end of visible
region (1.7 and 1.8 eV). This combination of attributes
indicates that this compound is a degenerate n-type
semiconductor, a property that has not previously
been observed in a Bi5+oxide.
Preparation
The preparation of magnesium bismuthate, MgBi2O6, was accomplished in a sealed PTFE-lined pressure vessel. The pressure vessel and contents were heated to and held at 135°C for 2.5 days. The pressure vessel was cooled to room temperature before opening. After separation of the solid by filtration and drying at 60°C for 24 h, a dark brown powder was obtained.