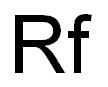Rutherfordium
- CAS No.
- 53850-36-5
- Chemical Name:
- Rutherfordium
- Synonyms
- Rutherfordium
- CBNumber:
- CB82520966
- Molecular Formula:
- Rf
- Molecular Weight:
- 261
- MDL Number:
- MOL File:
- 53850-36-5.mol
Rutherfordium Chemical Properties,Uses,Production
Physical properties
The chemical and physical properties of Unq (or rutherfordium) are homologous with theelement hafnium (72Hf ), located just above it in group 4 (IVB) in the periodic table. It wasfirst claimed to be produced artificially by the Joint Institute for Nuclear Research (JINR)located in Dubna, Russia. The Russian scientists used a cyclotron that smashed a target ofplutonium-242 with very heavy ions of neon-22, resulting in the following reaction: 94Pu-242 + 10Ne-22 →104Unq-260 + 40n-1 (alpha radiation). The Russians named Unq-260 “kurchatovium”(Ku-260) for the head of their center, Ivan Kurchatov. (See details in the next section,“History.”).
Isotopes
There are a total of 15 isotopes for rutherfordium, ranging from Rf-253 to Rf-264. Their half-lives range from 23 microseconds to 10 minutes. They are all artificiallymade, radioactive, and unstable. Their decay modes are a combination of alpha decayand spontaneous fission (SF).
Origin of Name
Named by the transition naming system of the IUPAC (unnilquadium) and later named after the New Zealand scientist Ernest Rutherford (rutherfordium).
History
Canadian, and British physicist); Rf; at. wt. [261]; at. no. 104.
In 1964, workers of the Joint Nuclear Research Institute at
Dubna (Russia) bombarded plutonium with accelerated 113
to 115 MeV neon ions. By measuring fission tracks in a special
glass with a microscope, they detected an isotope that decays
by spontaneous fission. They suggested that this isotope,
which has a half-life of 0.3 ± 0.1 s, might be 104.
Element 104, the first transactinide element, is expected to
have chemical properties similar to those of hafnium. It would,
for example, form a relatively volatile compound with chlorine
(a tetrachloride). The Soviet scientists have performed experiments
aimed at chemical identification, and have attempted
to show that the 0.3-s activity is more volatile than that of the
relatively nonvolatile actinide trichlorides. This experiment
does not fulfill the test of chemically separating the new element
from all others, but it provides important evidence for evaluation. New data, reportedly issued by Soviet scientists,
have reduced the half-life of the isotope they worked with
from 0.3 to 0.15 s. The Dubna scientists suggest the name
kurchatovium and symbol Ku for Element 104, in honor of
Igor Vasilevich Kurchatov (1903–1960), late Head of Soviet
Nuclear Research. The Dubna Group also has proposed the
name dubnium for Element 104. In 1969, Ghiorso, Nurmia,
Harris, K. A. Y. Eskola, and P. I. Eskola of the University of
California at Berkeley reported they had positively identified
two, and possibly three, isotopes of Element 104. The group
also indicated that after repeated attempts so far they have
been unable to produce isotope 260104 reported by the Dubna
groups in 1964. The discoveries at Berkeley were made by
bombarding a target of 249Cf with 12C nuclei of 71 MeV, and 13C
nuclei of 69 MeV. The combination of 12C with 249Cf followed
by instant emission of four neutrons produced Element 257104.
This isotope has a half-life of 4 to 5 s, decaying by emitting an
alpha particle into 253No, with a half-life of 105 s. The same
reaction, except with the emission of three neutrons, was
thought to have produced 258104 with a half-life of about 1/100
s. Element 259104 is formed by the merging of a 13C nuclei with
249Cf, followed by emission of three neutrons. This isotope has
a half-life of 3 to 4 s, and decays by emitting an alpha particle
into 255No, which has a half-life of 185 s. Thousands of atoms
of 257104 and 259104 have been detected. The Berkeley group
believes its identification of 258104 was correct. Eleven isotopes
of Element 104 have now been identified. The Berkeley
group proposed the name rutherfordium (symbol Rf) for the
new element, in honor of Ernest Rutherford. This name was
formally adapted by IUPAC in August 1997.
Uses
There are no uses for unnilquadium (rutherfordium), except for high-energy nuclear-particleresearch.
Definition
rutherfordium: Symbol Rf. A radioactivetransactinide element; a.n.104. It was first reported in 1964 atDubna, near Moscow, and in 1969 it was detected by A. Ghiorso and ateam at Berkeley, California. It canbe made by bombarding californium-249 nuclei with carbon-12 nuclei.
Definition
A radioactive metal not found naturally on earth. It is the first transactinide metal. Atoms of rutherfordium are produced by bombarding 249Cf with 12C or by bombarding 248Cm with 18O. Symbol: Rf; m.p. 2100°C (est.); b.p. 5200°C (est.); r.d. 23 (est.); most stable isotope 261Rf (half-life 65s).
Hazard
The hazards of unnilquadium are the same as for any radioactive element. Since there areonly a few atoms with short half-lives produced at a time in scientific laboratories, there islittle danger to the public.