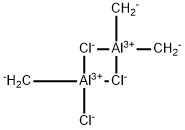
Trichlorotrimethyl dialuminum
- CAS No.
- 12542-85-7
- Chemical Name:
- Trichlorotrimethyl dialuminum
- Synonyms
- MASC;Trichlortrimethyldialuminium;trichlorotrimethyldialuminium;METHYLALUMINUM SESQUICHLORIDE;Trichlorotrimethyl dialuminum;Aluminum, trichlorotrimethyldi-;METHYLALUMINUMSESQUICHLORIDE(DOT);Methylaluminium sesquichloride, 1M solution in toluene
- CBNumber:
- CB9690059
- Molecular Formula:
- C3H6Al2Cl3
- Molecular Weight:
- 202.39
- MDL Number:
- MFCD00044876
- MOL File:
- 12542-85-7.mol
| Melting point | 23°C |
|---|---|
| Boiling point | 144°C |
| Density | 1.151 |
| form | liquid |
| EWG's Food Scores | 2 |
| FDA UNII | U57CQI9201 |
| EPA Substance Registry System | Aluminum, trichlorotrimethyldi- (12542-85-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H250-H314-H260 | |||||||||
| Precautionary statements | P210-P222-P280-P302+P334-P370+P378-P422-P223-P231+P232-P280-P335+P334-P370+P378-P402+P404-P501-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 | |||||||||
| RIDADR | 3052 | |||||||||
| HazardClass | 4.2 | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Trichlorotrimethyl dialuminum Chemical Properties,Uses,Production
Chemical Properties
The aluminum alkyl halides are flammable, reactive, and may be spontaneously combustible in air. They are colorless to yellow liquids. Ethylaluminum dichloride:(563-43-9):
Chemical Properties
Colorless liquid at 25C.
Uses
Catalyst for polymerization of olefins, catalyst for hydrogenation of aromatics.
Hazard
Ignites spontaneously in air, reacts violently with water, keep out of contact with air and moisture.
Safety Profile
A flammable liquid. Danger from spontaneous combustion. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
Shipping
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
Incompatibilities
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.
Trichlorotrimethyl dialuminum Preparation Products And Raw materials
Raw materials
Preparation Products
Trichlorotrimethyl dialuminum Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shanghai Orgchem Co.,Ltd. | +86-21-5877 1921 | info@chemofchina.com | China | 9661 | 55 |
| Shaanxi Dideu Medichem Co. Ltd | 029-61856359 18690052321 | 1027@dideu.com | China | 10011 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei xin bonus chemical co. LTD | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 58 |
| Mainchem Co., Ltd. | 55 |
| Shanghai Orgchem Co.,Ltd. | 55 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |