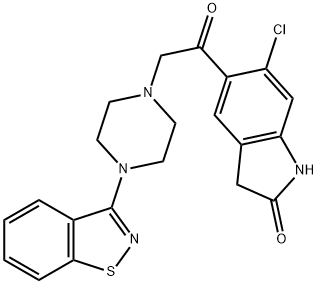
Ziprasidone QT
|
|
Ziprasidone QT 속성
- 끓는 점
- 601.8±55.0 °C(Predicted)
- 밀도
- 1.422±0.06 g/cm3(Predicted)
- 저장 조건
- Store at -20°C
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨, 가열)
- 산도 계수 (pKa)
- 12?+-.0.20(Predicted)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 연한 주황색에서 갈색까지
안전
Ziprasidone QT C화학적 특성, 용도, 생산
용도
Keto Ziprasidone is an intermediate in the synthesis of Hydroxy Ziprasidone which is an impurity of Ziprasidone (Z485000); a combined serotonin (5HT2) and dopamine (D2) receptor antagonist. Used as an antipsychotic.Ziprasidone QT 준비 용품 및 원자재
원자재
준비 용품
Ziprasidone QT 공급 업체
글로벌( 21)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 |
sales@molcore.com | China | 49739 | 58 |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 |
overseasales@yongstandards.com | China | 11927 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131981 | 58 |
| ChemStrong Scientific Co.,Ltd | 0755-0755-66853366 13670046396 |
sales@chem-strong.com | China | 17982 | 56 |
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 |
greensnown@163.com | China | 9911 | 50 |
| Artis Biotech Co. Ltd. | 19138486554 18582380095 |
sales@artisbio.com | China | 3066 | 58 |
| Hubei Yangxin Medical Technology Co., Ltd. | 15374522761 |
3003392093@qq.com | China | 7851 | 55 |
| Chengdu Novel Biomedical Co., Ltd. | 028-85255396 18302801538 |
cdnovell@163.com | China | 2238 | 58 |
| Shanghai Han-Xiang Chemical Co., Ltd. | 15971444841 |
amber@biochempartner.com | China | 3063 | 58 |
Ziprasidone QT 관련 검색:
1H-Indol-2-ol, 6-chloro-7-(2-chloroethyl)-
Ziprasidone Amino Acid
(Ziprasidone Impurity C)
3-(1,2-Benzisothiazolyl) Ziprasidone
(Ziprasidone Impurity E)
5,5'-Bis(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)-6,6'-dichloro-3-hydroxy-3,3'-biindoline-2,2'-dione
3-(1-Piperazinyl)-1,2-benzisothiazole
Ziprasidone N-Oxide
Hydroxy Ziprasidone
Ziprasidone IMpurity (6-Chloro-5-(2-Chloro-1-Hydroxy-Ethyl)-1,3-Dihydro-Indol-2-One)
Ziprasidone Sulfoxide
3-Oxo Ziprasidone
(Ziprasidone Impurity B)