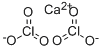염소산칼슘
|
|
염소산칼슘 속성
- 녹는점
- 340°C
- 밀도
- 2.71 g/cm3
- 물리적 상태
- 백색 결정
- 색상
- white crystals, crystalline
- 냄새
- 냄새 없는
- 수용성
- 197 at 25℃
- CAS 데이터베이스
- 10137-74-3
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 유엔번호(UN No.) | 1452 | ||
|---|---|---|---|
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 10137-74-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-04495 | ||
| 유해화학물질 필터링 | 97-1-198 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 염소산 염류 및 이를 1% 이상 함유한 혼합물. 다만, 폭발약은 제외 |
염소산칼슘 C화학적 특성, 용도, 생산
화학적 성질
Calcium chlorate forms white to yellow deliquescent crystals.물리적 성질
Calcium chlorate Ca(ClO3)2 is the chemical compound formed from calcium and the chlorate anion. Like KClO3, it is a strong oxidizer and can be used in pyrotechnic formulations. Its molecular weight is 206.98 g/mol. Its solubility in water is 209 g/100 ml at 20°C. It is slightly soluble in alcohol. Calcium chlorate has a melting point of 325°C and its density is 2.71 g/cm3. Its CAS number is 10017-74-3. A dihydrate, Ca(ClO3)2·2H2O, forms as white monoclinic crystals, which are decomposed by heating above 150°C. No structural data is available.용도
Calcium chlorate has been used as an herbicide, like sodium chlorate. It is occasionally used in photography, dusting powder to kill poison ivy, pyrotechnics, as an oxidizer and pink flame colorant. Its hygroscopic nature and incompatibility with other common pyrotechnic materials (such as sulfur) limit its utility in these applications.제조 방법
Calcium chlorate can be prepared by the Liebig process which is also used to prepare the alkali chlorates.In Liebig’s process, chlorine is passed into milk of lime, at or above a temperature of 1000°C, the apparent reaction being:
6Ca(OH)2+ 6Cl2→5CaCl2+ Ca(CIO3)2+ 6H2O.
But this may comprise two minor reactions:
2Ca (OH)2+ 2Cl2→CaCl2+ Ca(OCl)2+ 2H2O;
3Ca(OCI)2→2CaCl2+ Ca(CIO3)2
This salt can also be prepared from calcium chloride by an electrochemical method:
CaCl2 (aq)+ 6H2O (aq)+ e-→Ca(ClO3)2+ 6H2
using a platinum anode and a rotating stainless steel cathode. Yields of the dihydrate up to 78% have been achieved. The hydrogen produced can be “burned” to reform water.
일반 설명
Calcium chlorate appears as a white crystalline solid. It forms a very flammable mixture with combustible materials and this mixture may be explosive if the combustible material is finely divided. The mixture can be ignited by friction. Contact with strong sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. Prolonged exposure of the material to fire or heat can result in an explosion. Calcium chlorate is used in photography, in pyrotechnics, and as a herbicide.공기와 물의 반응
Water soluble.반응 프로필
An oxidizing agent. Liberates explosive chlorine dioxide gas in the presence of strong acid. Heating a moist mixture with a dibasic organic acid liberates chlorine dioxide and carbon dioxide. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806]. A combination with finely divided aluminum can explode by heat, percussion, or friction [Mellor 2:310 1946-47].위험도
Oxidizer, dangerous fire risk, forms explo- sive mixtures with combustible materials.건강위험
Inhalation of dust causes irritation of upper respiratory system. Dust irritates eyes and skin. Ingestion causes abdominal pain, nausea, vomiting, diarrhea, pallor, shortness of breath, unconsciousness.화재위험
Behavior in Fire: When involved in a fire, may cause an explosion. Irritating gases may be generated when heated.Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A powerful oxidant. Incompatible with Al, As, C, Cu, charcoal, MnO2, metal sulfides, S, dibasic organic acids, organic matter, P. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORATES for fire, disaster, and explosion hazards.잠재적 노출
Calcium chlorate is used in making fireworks, herbicides (weed killers) and in photography. Incompatibilities: A strong oxidizer. Reacts, possibly with risk of fire and explosion, with acids (especially organic acids), reducing agents; aluminum, arsenic, chemically active metals; combustible materials; ammonium compounds; charcoal, copper, cyanides; manganese dioxide, metal sulfides; phosphorus, sulfur운송 방법
UN1452 Calcium chlorate, Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN2429 Calcium chlorate, aqueous solution, Hazard Class: 5.1; Labels: 5.1-Oxidizer비 호환성
Calcium chlorate is used in making fireworks, herbicides (weed killers) and in photography. Incompatibilities: A strong oxidizer. Reacts, possibly with risk of fire and explosion, with acids (especially organic acids), reducing agents; aluminum, arsenic, chemically active metals; combustible materials; ammonium compounds; charcoal, copper, cyanides; manganese dioxide, metal sulfides; phosphorus, sulfur폐기물 처리
For barium chlorate, the UN recommends using a vast volume of a reducing agent (bisulfites, ferrous salts or hypo) followed by neutralization and flushing to the sewer with abundant water. This should be applicable here as well.염소산칼슘 준비 용품 및 원자재
원자재
준비 용품
염소산칼슘 공급 업체
글로벌( 5)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9116 | 58 |