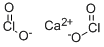Calcium chlorite
|
|
Calcium chlorite 속성
- 밀도
- 2.710
- 용해도
- reacts with H2O
- 색상
- white cubic crystals, crystalline
- 수용성
- H2O [HAW93]에 의해 분해됨
- CAS 데이터베이스
- 14674-72-7
안전
| 유엔번호(UN No.) | 1453 | ||
|---|---|---|---|
| 위험 등급 | 5.1 | ||
| 포장분류 | II |
Calcium chlorite C화학적 특성, 용도, 생산
개요
Calcium chlorite is an oxysalt with a molecular formula of Ca(ClO2)2. It is a white, crystalline material that is soluble in water. It is a strong oxidizer and a fire risk in contact with organic materials. The four-digit UN identification number is 1453. Hypochlorites have two less oxygen atoms than the base-state compounds. They can cause combustion in high concentrations when in contact with organic materials. When heated or in contact with water, they can give off oxygen gas. At ordinary temperatures, they can give off chlorine and oxygen when in contact with moisture and acids. They are commonly used as bleaches and swimming pool disinfectants.화학적 성질
White crystals. Decomposes in water.물리적 성질
Calcium chlorite, Ca(ClO2)2, has the molecular weight of 174.98 g/mol. Its density is 2.71 g/cm3. Its CAS number is 14674-72-7. Calcium chlorite can be prepared by reaction of chlorous acid with calcium carbonate:CaCO3 (solid)+ 2HClO2 (aq)→Ca(ClO2)2·6H2O
It is a white granular solid and if formed in solution, crystallizes as the hexahydrate. Calcium hypochlorite, hydrated, is a white granular solid having an odor of chlorine. It is noncombustible and accelerates the burning of combustible materials. It is decomposed by water with evolution of chlorine gas and heat. Prolonged exposure to fire or heat may result in an explosion. It is decomposed by water to form calcium hydroxide and chlorine dioxide, an explosive oxidizing gas:
Ca(ClO2)2+H2O→Ca(OH)2+ 2ClO2
Calcium chlorite is a strong oxidizing agent. It ignites on contact with potassium thiocyanate and reaction with chlorine yields explosive chlorine dioxide gas. Reaction with ammonia produces ammonium chlorite, which is shock sensitive and explodes. If mixed with finely divided metallic or organic substances, the combination is highly flammable and may be ignited by friction.
Currently, no manufacturer is registered for the product CALCIUM CHLORITE. There is a paucity of data concerning the physical properties of this salt and they are not easily accessible.
일반 설명
White granular solid. Prolonged exposure to fire or heat may result in an explosion and the rupturing of the containers.공기와 물의 반응
Decomposed by water to calcium hydroxide and chlorine dioxide, an explosive oxidizing gas.반응 프로필
A strong oxidizing agent. Accelerate the burning of combustible materials. Ignites on contact with potassium thiocyanate [Lewis]. Reaction with chlorine yields explosive chlorine dioxide gas. Reaction with ammonia produces ammonium chlorite, which is shock-sensitive. Finely divided mixtures with metallic or organic substances are highly flammable and may be ignited by friction (Lab. Gov. Chemist 1965).위험도
Strong oxidizer, fire risk in contact with organic materials.건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.Safety Profile
A strong oxidizer. Ignites on contact with potassium thiocyanate. Reaction with Cl2 yields explosive Cl0~. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORITES and CALCIUM COMPOUNDS.Calcium chlorite 준비 용품 및 원자재
원자재
준비 용품
Calcium chlorite 공급 업체
글로벌( 4)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
Calcium chlorite 관련 검색:
염소산칼슘 소듐클로라이트 염소산 스트론튬 차아염소산 칼슘 염소산 마그네슘 과염소산 칼슘
Barium hypochlorite
magnesium hypochlorite
BariumChlorite
MAGNESIUM CHLORITE
Dihypochlorous acid strontium salt
Bischlorous acid strontium salt
BERYLLIUM CHLORATE
CALCIUM PERCHLORATE
Calcium chlorate dihydrate
Calcium chlorite
CALCIUM PERCHLORATE HYDRATE