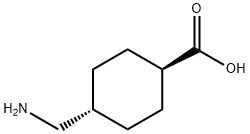
트라넥삼산
|
|
트라넥삼산 속성
- 녹는점
- >300 °C (lit.)
- 끓는 점
- 281.88°C (rough estimate)
- 밀도
- 1.0806 (rough estimate)
- 증기압
- 1.72hPa at 25℃
- 굴절률
- 1.4186 (estimate)
- 저장 조건
- 2-8°C
- 용해도
- 물과 빙초산에 잘 녹으며, 아세톤과 에탄올(96%)에는 거의 녹지 않습니다.
- 산도 계수 (pKa)
- pKa 4.3 (Uncertain);10.6 (Uncertain)
- 물리적 상태
- 결정성 분말
- 색상
- 하얀색
- 수용성
- 1g/6ml
- Merck
- 14,9569
- BRN
- 2207452
- 안정성
- 흡습성
- InChIKey
- GYDJEQRTZSCIOI-LJGSYFOKSA-N
- CAS 데이터베이스
- 1197-18-8
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-37/39 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | GU8400000 | ||
| 위험 등급 | IRRITANT | ||
| HS 번호 | 29224999 | ||
| 독성 | LD50 in mice, rats (mg/kg): 1500, 1200 i.v. (Melander) | ||
| 기존화학 물질 | KE-01455 |
트라넥삼산 C화학적 특성, 용도, 생산
개요
Tranexamic acid is a derivative of aminomethylbenzoic acid, and a kind of antifibrinolytic drugs to stop bleeding. The hemostasis mechanism of tranexamic acid is similar to aminocaproic acid and aminomethylbenzoic acid, but the effect is stronger. The strength is 7 to 10 times of aminocaproic acid, 2 times of aminomethylbenzoic acid, but toxicity is similar.The chemical structure of tranexamic acid is similar to lysine, competitive inhibition of plasmin original in fibrin adsorption, to prevent their activation, protection fiber protein not to degrade by plasmin and dissolve, eventually achieve hemostasis. Applicable in the treatment of acute or chronic, localized or systemic primary fiber fibrinolytic hyperthyroidism caused by bleeding, such as obstetric hemorrhage, renal hemorrhage, hemorrhage of hypertrophy of the prostate, hemophilia, pulmonary tuberculosis hemoptysis, stomach bleeding, after operation of liver, lung, spleen and other viscera hemorrhage; also can be used in surgery when abnormal bleeding etc..
Clinical tranexamic acid has effect significantly to insect bites disease, dermatitis and eczema, simple purpura, chronic urticaria, artificial sex urticaria, toxic eruption and eruption. And also has a certain effect on erythroderma, scleroderma, systemic lupus erythematosus (SLE), Erythema multiforme, shingles and alopecia areata. Treatment of hereditary angioedema effect is also good. In the treatment of Chloasma, general medicine is effective about 3 weeks, markedly effective 5 weeks, a course of 60 days. Given orally in doses of 0.25 ~ 0.5 g, a day 3 ~ 4 times. A few patients can nausea, fatigue, pruritus, abdominal discomfort, and diarrhea side effects after withdrawal symptoms disappear.
화학적 성질
Tranexamic acid is a White or almost white, crystalline powder. It is freely soluble in water and in glacial acetic acid and is very slightly soluble in ethanol and practically insoluble in ether.용도
Fibrinolysis, the cleavage of fibrin by plasmin, is a normal step in the dissolution of fibrin clots after wound repair. Tranexamic acid is an inhibitor of fibrinolysis that blocks the interaction of plasmin with fibrin (IC50 = 3.1 μM). It is a lysine mimetic that binds the lysine binding site in plasmin. Antifibrinolytic agents have value when fibrinolytic activity is abnormally high or when coagulation is impaired.정의
ChEBI: Tranexamic acid is a monocarboxylic acid. It has a role as an antifibrinolytic drug and a hematologic agent. It is functionally related to a cyclohexanecarboxylic acid.일반 설명
Tranexamic acid is an antifibrinolytic agent and is commonly used for heavy menstrual bleeding.Mechanism of action
Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the lysine receptor binding sites of plasmin for fibrin are occupied, preventing binding to fibrin monomers, thus preserving and stabilizing fibrin’s matrix structure. This agent has a longer half-life, is approximately ten times more potent, and is less toxic than aminocaproic acid, which possesses similar mechanisms of action.부작용
Tranexamic acid is mostly well-tolerated.Common
nausea (upset stomach)
vomiting (throwing up)
diarrhea
headache
Occasional
dizziness
giddiness
vision changes
Rare
stroke
blood clots in undesired areas
deep vein thrombosis
참고 문헌
Intravenous use of tranexamic acid reduces postoperative blood loss in total knee arthroplasty DOI:10.1007/s00402-014-2081-xwww.childrensmn.org
dermnetnz.org
Tranexamic acid for the prevention and treatment of postpartum haemorrhage. DOI:10.1097/01.aoa.0000552886.12061.3c
Sittig's Pharmaceutical Manufacturing Encyclopedia
The Renal Drug Handbook
Synthesis of Essential Drugs (2006, Elsevier) - libgen.lc
Spectrophotometric and spectrofluorimetric methods for the determination of tranexamic acid in pharmaceutical formulation. DOI:10.1248/CPB.55.364
Determination of plasma tranexamic acid using cation-exchange high-performance liquid chromatography with fluorescence detection. DOI:10.1016/S0378-4347(00)84573-9
[1] A Pilbrant, J Vessman, M Schannong. “Pharmacokinetics and bioavailability of tranexamic acid.” European Journal of Clinical Pharmacology 20 1 (1981): 65–72.
트라넥삼산 준비 용품 및 원자재
원자재
수산화바륨
4-(Aminomethyl)benzoic acid
수소
4-(Acetylamino-methyl)-cyclohexanecarboxylic acid methyl ester
Methyl 4-cyanobenzoate
(1r,4r)-methyl 4-cyanocyclohexanecarboxylate
trans-4-cyanocyclohexane-1-ylcarboxylic acid
준비 용품
트라넥삼산 공급 업체
글로벌( 953)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Beri Pharma Co., Ltd. | +8613417710166 |
sales@zhberi.com | China | 227 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 |
gksales1@gk-bio.com | China | 9316 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 50003 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 973 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +8618580541567 |
sales@zhswyy.com | China | 303 | 58 |
| Wuhan Fortuna Chemical Co.,Ltd | +8618007136271 |
hk@fortunachem.com | China | 5984 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3878 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 |
sales06@tgybio.com | China | 905 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
트라넥삼산 관련 검색:
트라넥삼산 메틸사이클로헥산 사이클로헥산카본산
BOC-(4-AMINOMETHYL)-CYCLOHEXANE-CARBOXYLIC ACID
Tranexamic acid
TRANEXAMIC ACID IMPURITY C
trans-4-Methylcyclohexanecarboxylic acid
cis,trans-(1,1-DiMethylethoxy)carbonyl TranexaMic Acid-13C2,15N
Tranexamic acid impurity F
TranexaMic acid iMpurity B
Tranexamic acid impurity E
TranexaMic Acid IMpurity D
(2S,4R)-4-(thiophen-3-ylMethyl)pyrrolidine-2-carboxylic acid hydrochloride
trans-4-(3-Phenylallyl)-L-proline hydrochloride
(2S,4R)-4-(4-cyanobenzyl)pyrrolidine-2-carboxylic acid hydrochloride
(2S,4R)-4-(3-fluorobenzyl)pyrrolidine-2-carboxylic acid hydrochloride
BOC-N-METHYL-TRANEXAMIC ACID
trans-4-(azidomethyl)cyclohexanecarboxylic acid