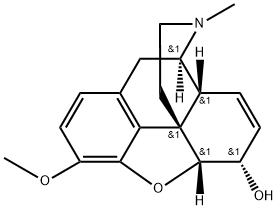
코데인
|
|
코데인 속성
- 녹는점
- 156-158?C
- 끓는 점
- 440.69°C (rough estimate)
- 밀도
- 1.3200
- 굴절률
- 1.5500 (estimate)
- 인화점
- 9℃
- 저장 조건
- 2-8°C
- 용해도
- 끓는 물에 용해되며 에탄올(96%)에 잘 용해됩니다.
- 산도 계수 (pKa)
- 8.21(at 25℃)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 옅은 갈색까지
- 수용성
- 47.62g/L(25℃)
- 안정성
- 안정적이지만 빛에 민감합니다. 타기 쉬운. 강한 산화제, 브롬화물, 요오드화물, 중금속 염과 호환되지 않습니다.
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-23/24/25-39/23/24/25 | ||
| 안전지침서 | 7-16-36/37-45 | ||
| 유엔번호(UN No.) | UN 1230 3/PG 2 | ||
| WGK 독일 | 1 | ||
| 유해 물질 데이터 | 76-57-3(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: 427mg/kg |
코데인 C화학적 특성, 용도, 생산
개요
Codein phosphate and hydrochloride caused contact dermatitis in a worker in the production of opium alkaloids. Codeine bitartrate caused contact dermatitis in a worker in the production of concentrated poppy straw. Codeine was a sensitizer in a laboratory worker at an opiate-manufacturing pharmaceutical company, also sensitive to the baine.화학적 성질
Codeine, also known as methylmorphine, C18H21NO3· H2O, is a colorless white crystalline substance, slightly soluble in water, soluble in alcohol and chloroform, effloresces slowly in dry air.용도
Codeine is used in medicine for its narcoticanalgesic action. It is used as a sedative incough mixtures. Codeine occurs in opiumfrom 0.7% to 2.5%. It is prepared frommorphine by methylating the phenolic OHgroup of the morphine. It is also obtained byextraction of opium.정의
codeine: An alkaloid C18H21NO3found in opium. It is structurally similarto morphine, from which it isproduced, and is used in the form ofthe sulphate or phosphate as apainkiller and cough medicine.Codeine is converted into morphinein the liver. It is used to some extentas a recreational drug. In the UK it isa class B drug but can be obtained incomposite over-the-counter preparationsin which it has a low concentrationand is combined with paracetamolor ibuprofen. See also opioids.World Health Organization (WHO)
Codeine, which has antitussive, opioid analgesic and antidianhoeal activity, was first extracted from opium in 1832 and has since been widely used in medicine. The development of dependence and its potential for abuse resulted in the control af the substance under Schedule II of the 1961 Single Convention on Narcotic Drugs. Preparations containing codeine remain widely available and are included in the WHO Model List of Essential Drugs.(Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert Committee, 722, , 1985)Biological Functions
Like morphine, codeine is a naturally occurring opioid found in the poppy plant. Codeine is indicated for the treatment of mild to moderate pain and for its antitussive effects. It is widely used as an opioid antitussive because at antitussive doses it has few side effects and has excellent oral bioavailability. Codeine is metabolized in part to morphine, which is believed to account for its analgesic effect. It is one of the most commonly used opioids in combination with nonopioids for the relief of pain. The administration of 30 mg of codeine in combination with aspirin is equivalent in analgesic effect to the administration of 65 mg of codeine. The combination of the drugs has the advantage of reducing the amount of opioid required for pain relief and abolition of the pain via two distinct mechanisms, inhibition of prostanoid synthesis and opioid inhibition of nociceptive transmission. When given alone, orally administered codeine has about one-tenth to one-fifth the potency of morphine for the relief of pain. In addition, IV codeine has a greater tendency to release histamine and produce vasodilation and hypotension than does morphine. Thus, the use of IV codeine is rare. Codeine is rarely addictive and produces little euphoria.일반 설명
Codeine is an alkaloid that occurs naturally in opium, butthe amount present is usually too small to be of commercialimportance. Consequently, most commercial codeine is preparedfrom morphine by methylating the phenolic OHgroup. It occurs as levorotatory, colorless, efflorescent crystals,or as a white crystalline powder. It is light sensitive.Codeine is a monoacidic base and readily forms salts withacids, with the most important being the sulfate and thephosphate. The acetate and methylbromide derivatives havebeen used to a limited extent in cough preparations.The general pharmacological action of codeine is similarto that of morphine, but it does not possess the sameanalgesic potency.공기와 물의 반응
CODEINE is light sensitive and sensitive to prolonged exposure to air. Insoluble in water.반응 프로필
CODEINE is incompatible with bromides, iodides and salts of heavy metals. . CODEINE is an amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.위험도
Habit-forming narcotic, sale legally restricted.건강위험
The toxic effects due to codeine are similarbut less toxic than those of morphineand other opium alkaloids. An overdosecan cause respiratory failure. It is a weakdepressant of the central nervous system.It also exhibits stimulant action. Toxicsymptoms from high dosages may includedrowsiness, sleep, tremors, excitement, andhallucinations. It may also produce gastricpains and constipation. An oral LD50 value in rats is 427 mg/kg. The habit-forming effectsof codeine are lower than those associatedwith morphine.Nagamatsu and coworkers (1985) havereported in vitro formation of codeinonefrom codeine by rat or guinea pig liverhomogenate. Codeinone may be a metabolicintermediate in the presence of nicotinamideadenine dinucleotide (NAD). Its acute toxicityin mice was determined to be 30 timeshigher than that of codeine.
화재위험
CODEINE is combustible.색상 색인 번호
Codeine has been reported as an occupational sensitizer in workers in the production of opium alkaloids. Codeine has been responsible for fixed drug eruptions or generalized dermatitis. Cross-sensitivity is expected to morphine.Pharmacology
Codeine is a constituent of opium. Up to 10% of a dose of codeine is metabolised by the hepatic microsomal enzyme CYP2D6 to morphine, which contributes significantly to its analgesic effect. The rest is metabolised in the liver to norcodeine and then conjugated to produce glucuronide conjugates of codeine, norcodeine and morphine. Codeine is considerably less potent than morphine. A round 8% of Western Europeans are deficient in the CYP2D6 enzyme and may not experience adequate analgesia with codeine. S imilarly, with super-metabolisers, there may be problems with opioid toxicity; particular care is needed in the breastfeeding mother as morphine is transferred in milk. Codeine can cause significant histamine release, and intravenous administration should be avoided. It has marked antitussive effects and also causes significant constipation. It is often combined with paracetamol.Purification Methods
Codeine crystallises from water or aqueous EtOH. Dry it at 80o. Evaporation of a CHCl3 extract gives a colourless glass which crystallises on scratching. It crystallises from H2O as the monohydrate, m 157-158.5o, and has [] D -136o (c 2.8, EtOH). The hydrobromide crystallises in needles from H2O, and effervesces at 151-160o, solidifies and remelts with extensive decomposition at 273-278o. It sublimes at 100o/0.03mm. [Gates J Am Chem Soc 75 4340 1953, Dauben et al. J Org Chem 44 1567 1979, Beilstein 27 II 136, 27 III/IV 2228.]코데인 준비 용품 및 원자재
원자재
준비 용품
코데인 관련 검색:
옥시코돈 5,5-디메틸-1,3-사이클로헥사디온 5,5-디알릴바비터 산 헤로인
CODEINE HYDROCHLORIDE
CODEINE PHOSPHATE
CODEINE SULFATE OPIATE ANALGESIC, ANT
ETHYLMORPHINE
CODEINE
2-CHLORO-4-ISOPROPROXYPHENYLBORONIC ACID
HYDROCODONE
R(-)-DENOPAMINE
Desogestrel
Desonide
R(-)-APOCODEINE HYDROCHLORIDE
2-PHTHALIMIDOETHYL ACETATE
OXYCODONE HYDROCHLORIDE
NORCODEINE