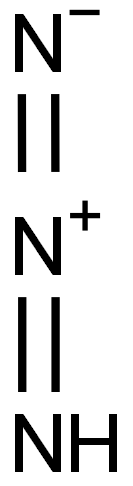Hydrazoic acid
|
|
Hydrazoic acid 속성
- 녹는점
- -80°
- 끓는 점
- bp 37°
- 밀도
- 1.092
- 용해도
- soluble in H2O
- 산도 계수 (pKa)
- 4.72(at 25℃)
- 물리적 상태
- 무색의 액체
- 색상
- colorless liquid; explodes, explosive
- 수용성
- 매우 잘 녹는 H2O [HAW93]
- 노출 한도
- Ceiling 0.1 ppm vapor (ACGIH).
- 안정성
- 불안정한. 농축되거나 순수한 상태에서는 격렬한 폭발이 일어납니다. 중금속과 쉽게 폭발성 화합물을 형성합니다. 이는 위험하고 취급하기 어려운 물질이므로 전문가가 아닌 사람이 준비하거나 취급해서는 안 됩니다.
안전
| 유엔번호(UN No.) | 0473 | ||
|---|---|---|---|
| 위험 등급 | 1.1A | ||
| 유해 물질 데이터 | 7782-79-8(Hazardous Substances Data) | ||
| 독성 | LD50 in mice (mg/kg): 21.5 i.p. (Graham) | ||
| 기존화학 물질 | KE-20179 |
Hydrazoic acid C화학적 특성, 용도, 생산
개요
Hydrazoic acid or hydrogen azide is a dangerous explosion risk when shocked or heated. It is the gas-forming agent in many air bag systems in automobiles and escape chutes in airplanes.화학적 성질
Colorless, volatile liquid; obnoxious odor.Soluble in water.물리적 성질
Colorless, volatile liquid; pungent disagreeable odor; density 1.09 g/mL;solidifies at -80°C; boils at 37°C; highly soluble in water; soluble in alkalies,alcohol and ether; pKa4.6 at 25°C.용도
Hydrazoic acid is used in making heavymetal azides for detonators. It forms readilywhen sodium azide reacts with acid orhydrazine is mixed with nitrous acid.정의
A colorless liquid with a nauseating smell. It is highly poisonous and explodes in the presence of oxygen and oxidizing agents. It can be made by distilling a mixture of sodium azide (NaN3) and a dilute acid. It is usually used as an aqueous solution. The salts of hydrazoic acid (azides), especially lead azide (Pb(N3)2), are used in detonators because of their ability to explode when given a mechanical shock.생산 방법
Hydrazoic acid is formed (1) by reaction of sodium nitrate with molten sodamide, (2) by reaction of nitrous oxide with molten sodamide, (3) by reaction of nitrous acid and hydrazinium ion (N2H5 + ), (4) by oxidation of hydrazinium salts, (5) by reaction of ethyl nitrite with NaOH solution and acidifying.제조 방법
Hydrazoic acid is prepared by reacting sulfuric acid with sodium azide:H2SO4 + NaN3 → HN3 + Na2SO4
or by treating hydrazine with nitrous acid:
N2H4 + HNO2 → HN3 + 2H2O
or by heating sodium amide with nitrous oxide:
NaNH2 + N2O → HN3 + NaOH
화학 반응
Hydrazoic acid reacts (1) with metals, e.g., magnesium, aluminum, zinc, iron, to form azides or hydrazoates (or trinitrides), (2) with heavy metal salt solutions to form insoluble azides, e.g., silver azide AgN3, mercury(I) azide HgN3, lead azide PbN6. Silver, mercury(I), and copper(I) azides decompose in the light to form nitrogen plus the metal. (3) It reacts with NH4OH to form ammonium azide NH4·N3, (4) with hydrazine to form hydrazine azide N2H4·HN3, (5) with sodium hypochlorite plus acetic acid to form chlorazide ClN3, explosive, (6) with sodium amalgam to form NH3 with some hydrazine, (7) with potassium permanganate to form nitrogen and H2O.위험도
Dangerous explosion risk when shocked or heated. Strong irritant to eyes and mucous membranes.건강위험
The acute toxicity of hydrazoic acidthrough inhalation and other routes of exposurehas been found to be high to very high.The symptoms and the intensity of poisoningare similar to sodium azide. It is, however,less toxic than hydrogen cyanide. Inhumans, inhalation of its vapors can produceirritation of eyes and respiratory tract, bronchitis,headache, dizziness, weakness, anddecreased blood pressure (Matheson 1983).Prolonged exposure to high concentrationscan result in collapse, convulsion, and death.An exposure to 1100 ppm for 1 hour waslethal to rats. Chronic exposure to a lowlevel of this compound in air may producehypotension.Animals given intraperitoneal dosages ofhydrazoic acid showed the symptoms ofheavy breathing, convulsions, depression,and fall in blood pressure. It affected thecentral nervous system, but no damage wasobserved in the liver or kidney.
LD50 value, intraperitoneal (mice): 22 mg/kg.
화재위험
In pure form or highly concentrated solution, hydrazoic acid is a dangerous explosive compound. It is unstable and sensitive to heat and shock. The explosion hazard decreases significantly with more dilute solutions.It forms shock-sensitive metal azides when react with metal salts, and fluorine azide with fluorine (Lawless and Smith 1968) and susceptible to form chlorine azide and bromine azide with chlorine gas and bromine vapor. All these products can explode violently on impact. With carbon disulfide it forms a violently explosive salt (Mellor 1946; NFPA 1997).
폐기물 처리
Hydrazoic acid may be destroyed by convertingit to sodium azide. The latter isdecomposed with nitrous acid in a hood(National Research Council 1995). The followingmethod is used. It is diluted in waterto a strength below 5%; or its solution inorganic solvents that is immiscible in wateris shaken vigorously with water in a separatoryfunnel. The aqueous solution containinghydrazoic acid is neutralized with sodiumhydroxide and separated from any organiclayer. Sodium azide, so formed, is destroyedby reacting the aqueous solution with anexcess of sodium nitrite followed by 20%sulfuric acid until the solution is acidic. Thereaction is carried out in a three-necked flaskequipped with a stirrer, a dropping funnel,and a gas outlet line to vent out nitric oxide.The reaction mixture is flushed down thedrain.Hydrazoic acid 준비 용품 및 원자재
원자재
준비 용품
Hydrazoic acid 공급 업체
글로벌( 6)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9320 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 |
1046@dideu.com | China | 10008 | 58 |
Hydrazoic acid 관련 검색:
지도부딘 소디움 아지이드 아지도트리메틸실란 2,6-비스(4-아지도벤질이덴)-4-메틸사이클로헥사논 바륨 아지드 디페닐포스포릴아지드
AZIDOMETHYL PHENYL SULFIDE
BENZYL AZIDE
4-METHOXYBENZYLOXYCARBONYL AZIDE
4-Acetamidobenzenesulfonyl azide
PHENYL AZIDE
4-AZIDOBENZOIC ACID
Trityl azide
DIPHENYLPHOSPHINYL AZIDE
4-FLUORO-3-NITROPHENYL AZIDE
8-Azidoadenosine
DIPHENYLMETHYLAZIDOSILANE
TRIPHENYLSILYLAZIDE