인화칼슘 C화학적 특성, 용도, 생산
개요
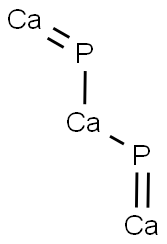
Calcium phosphide has the molecular formula of
Ca3P2 and the molecular weight of 182.1825 g/mol. Its
CAS number is 1305-99-3. It is a red-brown crystalline
material with a melting point of 1605°C. Its density is
2.51 g/cm
3. It readily reacts with water to form phosphine,
PH3, but is insoluble in ethanol.
It is easily prepared by reacting the metal with red
phosphorus at high temperature. The best way is to
sublime the P4 at 450°C in an inert gas stream and react
it with Mg metal at 750°C:
6Ca + 2P4?2Ca3P4
화학적 성질
Calcium phosphide is a gray granular solid or
reddish-brown crystalline solid. It has a musty odor, somewhat like acetylene.
용도
Metal phosphides, primarily Ca3P2, have been used
as rodenticides. Calcium phosphide baits have strong,
pungent garlic-like odor characteristic for phosphine
liberated by hydrolysis. The odor attracts rodents, but
has a repulsive effect on other animals, who are not
receptive to the smell. This salt has uses in incendiary
bombs and other explosives. On contact with acids or
water, calcium phosphide releases phosphine, which
ignites spontaneously. It is also used in fireworks, torpedoes,
self-igniting naval pyrotechnic flares, and various
water-activated ammunition.
일반 설명
CALCIUM PHOSPHIDE appears as red-brown crystals to gray granular lumps. CALCIUM PHOSPHIDE reacts with water to form calcium hydroxide and phosphine, a flammable poisonous gas. Phosphine will normally ignite spontaneously in contact with air. If there is an excess of water this fire of phosphine will not normally ignite surrounding combustible material.
반응 프로필
CALCIUM PHOSPHIDE and hydrochloric acid undergo a very energetic reaction [Mellor 8:841 1946-47]. Calcium and other alkaline earth phosphides incandesce in oxygen when heated.
위험도
Dangerous fire risk; decomposed by water
to phosphine, which is highly toxic and flammable.
See phosphine.
건강위험
Inhalation or ingestion causes faintness, weakness, nausea, vomiting. External contact with dust causes irritation of eyes and skin.
화재위험
Behavior in Fire: Can cause spontaneous ignition if wet. Contributes dense smoke of phosphoric acid.
Safety Profile
Highly toxic due to
phosphde, which in presence of moisture
emits phosphine. The phosphine may ignite
spontaneously in air. Incandescent reaction
with oxygen at 300°C. Incompatible with
dichlorine oxide. When heated to
decomposition it emits toxic fumes of POx.
See also CALCIUM COMPOUNDS and
PHOSPHIDES.
잠재적 노출
A strong reducing agent. Forms spontaneously combustible phosphine gas in moist air. Contact
with water or acids release phosphine gas, and can cause
explosions. Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides, chlorine monoxide, halogens, halogen
acids, oxygen, sulfur
운송 방법
UN1360 Calcium phosphide, Hazard Class: 4.3;
Labels: 4.3-Dangerous when wet material
비 호환성
A strong reducing agent. Forms spontaneously combustible phosphine gas in moist air. Contact
with water or acids release phosphine gas, and can cause
explosions. Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides, chlorine monoxide, halogens, halogen
acids, oxygen, sulfur
폐기물 처리
Disposal of unused product
must be undertaken by qualified personnel who are
knowledgeable in all applicable regulations and follow all
pertinent safety precautions including the use of appropriate protective equipment. For proper handling and disposal, always comply with federal, state, and local
regulations
인화칼슘 준비 용품 및 원자재
원자재
준비 용품