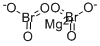마그네슘 브로메이트
|
|
마그네슘 브로메이트 속성
안전
| 유엔번호(UN No.) | 1473 | ||
|---|---|---|---|
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| 기존화학 물질 | KE-22684 |
마그네슘 브로메이트 C화학적 특성, 용도, 생산
개요
Magnesium bromate hexahydrate is a white crystalline compound that is insoluble in alcohol, but soluble in water. It reacts with methyl alcohol to form a complex. It is a fire hazard but is used as an analytical reagent.Magnesium bromate has found little usage in Industry because there are better oxidizing agents available. It is offered for sale commercially but in limited quantities.화학적 성질
White crystals or crystalline powder. Soluble in water; insoluble in alcohol.용도
Analytical reagent, oxidizing agent.제조 방법
Magnesium Bromate can be prepared by the reaction of bromic acid and magnesium carbonate:MgCO3+ 2HBrO3→Mg(BrO3)2+ CO2+H2O
However, magnesium bromate is not stable in an acidic environment and tends to decompose if one attempts to remove it from solution. Thus, the best method of preparation involves the use of sodium bromate which is basic enough to prevent such decomposition in solution:
Mg(OH)2+ 2NaBrO3→Mg(BrO3)2+ 2NaOH
The product is a hexahydrate, Mg(BrO3)2·6H2O This salt can also be prepared by adding a solution of magnesium sulfate to a suspension of barium bromate:
MgSO4 (aq)+ Ba(BrO3)2 (s)→Mg(BrO3)2 (aq)+ BaSO4(s)
The solution is then evaporated to recrystallize the hexahydrate, Mg(BrO3)2·6H2O.
일반 설명
Crystalline solid. Soluble in water and denser than water. Hence sinks in water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.공기와 물의 반응
Soluble in water.반응 프로필
MAGNESIUM BROMATE is an oxidizing agent. May cause ignition in contact with organic materials. A combination of finely divided aluminum with finely divided MAGNESIUM BROMATE can explode by heat, percussion, or friction [Mellor 2:310. 1946-47].위험도
Dangerous fire risk in contact with organic materials.건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.마그네슘 브로메이트 준비 용품 및 원자재
원자재
준비 용품
마그네슘 브로메이트 공급 업체
글로벌( 18)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9357 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 8132 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-87576359 15353716720 |
1039@dideu.com | China | 10011 | 58 |
| Guangdong Qianjin Chemical Reagent Co., Ltd. | 0751-2818792 13435114148 |
3128240096@qq.com | China | 2996 | 58 |