Glyoxylic acid suppliers
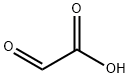
Glyoxylic acid
- CAS:
- 298-12-4
- MF:
- C2H2O3
- MW:
- 74.04
Suppliers by country/region
Company Type
Properties
- Melting point:
- -93°C
- Boiling point:
- 111°C
- Density
- 1.33 g/mL at 20 °C
- vapor pressure
- 14hPa at 19.85℃
- refractive index
- n
20/D 1.414
- Flash point:
- 111°C
- storage temp.
- Store below +30°C.
- solubility
- Miscible with ethanol. Slightly miscible with ether and benzene. Immiscible with esters.
- pka
- 3.18(at 25℃)
- form
- clear liquid
- color
- Colorless to Light orange to Yellow
- Water Solubility
- miscible
- Merck
- 14,4511
- BRN
- 741891
- InChIKey
- HHLFWLYXYJOTON-UHFFFAOYSA-N
- LogP
- -0.930 (est)
Safety Information
- Symbol(GHS)


GHS05,GHS07
- Signal word
- Danger
- Hazard statements
- H290-H317-H318
- Precautionary statements
- P234-P261-P272-P280-P302+P352-P305+P351+P338
- Hazard Codes
- C,Xi
- Risk Statements
- 34-43-41
- Safety Statements
- 26-36/37/39-45-37/39-24
- RIDADR
- UN 3265 8/PG 2
- WGK Germany
- 1
- RTECS
- MD4550000
- TSCA
- Yes
- HazardClass
- 8
- PackingGroup
- III
- HS Code
- 29183000
- Toxicity
- rat,LDLo,intramuscular,250mg/kg (250mg/kg),Bolletino della Societe Italiana di Biologia Sperimentale. Vol. 36, Pg. 1937, 1960.
Use
Glyoxylic acid has been employed as reducing agent in electroless copper depositions by free-formaldehyde method in synthesis of new chelating agent, 2-(2-((2-hydroxybenzyl)amino)ethylamino)-2-(2-hydroxyphenyl)acetic acid (DCHA).
361 supplier list of "Glyoxylic acid"
$225.00-65.00 / 1Kg/Bag
- Product Name:Glyoxylic Acid monohydrate
- Products Intro:Purity: 99% up, High Density|Package: 1Kg/Bag;225.00;USD|25Kg/Bag;125.00;USD|100Kg/Bag;65.00;USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: APIs,pharmaceutical intermediates,health and food supplements,cosmetic raw materials,herbal extracts
$10.00/ 200Kg/Drum
- Product Name:Glyoxylic acid
- Products Intro:Purity: 99.5% | Package: 200Kg/Drum;10USD | CustNote: /
- Company Type: Trader
- Country/Region: CHINA
- Main Products: DHA,4'-Methylpropiophenone,Procaine hcl,tetramisole hcl,lidocaine
$180.00/ 1kg
- Product Name:Glyoxylic Acid 50
- Products Intro:Purity: 99% | Package: 1kg;180USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: Candelilla Wax,Boric acid,Helional,carbomer,1,3-Dihydroxyacetone
- Product Name:Glyoxylic acid
- Products Intro:Purity: 995 | Package: 200Kg/Drum;USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: Carbohydrazide,o-Phthalaldehyde,4-hydroxy tempo,Sodium tetraphenylborate,Terephthalaldehyde
$1.00/ 1Kg/Drum
- Product Name:GLYOXYLIC ACID
- Products Intro:Purity: 50% | Package: 1Kg/Drum;1USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: acetic acid,Ethyl oleate,Hypophosphorous acid,tetramisole,OPP
$0.00-0.00 / 1KG
- Product Name:Glyoxylic acid
- Products Intro:Purity: 99% | Package: 1KG;USD|250KG;USD|1000KG;USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: Dimethyl sulfoxide,Bromobenzene,Tetrabromobisphenol A,1,4-Dichlorobenzene,Diethylamine
$9.00/ 25kg
- Product Name:Glyoxylic Acid
- Products Intro:Purity: 50% | Package: 25kg;9USD
- Company Type: Trader
- Country/Region: CHINA
- Main Products: Colophony,Urea,Paraffin Wax,Glycerin,SLES