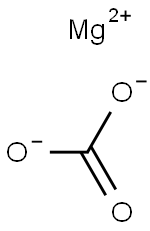
Magnesium carbonate
|
- $39 - $235
- Product name: Magnesium carbonate
- CAS: 546-93-0
- MF: CMgO3
- MW: 84.31
- EINECS:208-915-9
- MDL Number:MFCD00064632
- Synonyms:carbonicacid,magnesiumsalt ;Carbonicacid,magnesiumsalt(1:1) ;ci77713 ;DCI Light Magnesium Carbonate;dcilightmagnesiumcarbonate ;barringtonite ;C.I. 77713;c.i.77713
2 prices
Selected condition:
Brand
- AK Scientific
- TRC
Package
- 100g
- ManufacturerAK Scientific
- Product numberC663
- Product descriptionMagnesium carbonate
- Packaging100g
- Price$39
- Updated2021-12-16
- Buy
- ManufacturerTRC
- Product numberM110325
- Product descriptionMagnesium carbonate
- Packaging100g
- Price$235
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| AK Scientific | C663 | Magnesium carbonate | 100g | $39 | 2021-12-16 | Buy |
| TRC | M110325 | Magnesium carbonate | 100g | $235 | 2021-12-16 | Buy |
Properties
Melting point :990°C
Density :3.050
solubility :Practically insoluble in water. It dissolves in dilute acids with effervescence.
form :Solid
color :White
Odor :at 100.00?%. odorless
Water Solubility :g MgCO3/100g solution at CO2 pressure, kPa, 18°C: 3.5 (203), 4.28 (405), 5.90 (1010), 7.49 (1820), 7.49 (5670); at 0°C 8.58 (3445), at 60°C 5.56 (3445); soluble acids; insoluble alcohol [HAW93] [KIR81]
Stability :Hygroscopic
LogP :-0.809 (est)
CAS DataBase Reference :546-93-0(CAS DataBase Reference)
NIST Chemistry Reference :Magnesium carbonate(546-93-0)
EPA Substance Registry System :Magnesium carbonate (546-93-0)
Density :3.050
solubility :Practically insoluble in water. It dissolves in dilute acids with effervescence.
form :Solid
color :White
Odor :at 100.00?%. odorless
Water Solubility :g MgCO3/100g solution at CO2 pressure, kPa, 18°C: 3.5 (203), 4.28 (405), 5.90 (1010), 7.49 (1820), 7.49 (5670); at 0°C 8.58 (3445), at 60°C 5.56 (3445); soluble acids; insoluble alcohol [HAW93] [KIR81]
Stability :Hygroscopic
LogP :-0.809 (est)
CAS DataBase Reference :546-93-0(CAS DataBase Reference)
NIST Chemistry Reference :Magnesium carbonate(546-93-0)
EPA Substance Registry System :Magnesium carbonate (546-93-0)
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Magnesium carbonate is obtained mainly by mining the natural mineral magnesite. The trihydrate salt, MgCO3·3H2O, is prepared by mixing solutions of magnesium and carbonate ions in the presence of carbon dioxide.More related product prices
MAGNESIUM CARBONATE Sodium carbonate Magnesium sulfate Calcium carbonate Dimethyl carbonate Magnesium sulfate heptahydrate 39409-82-0 MAGNESIUM CARBONATE Guanidine carbonate Strontium carbonate Cesium carbonate Manganese carbonate Lithium carbonate LEAD CARBONATE CADMIUM CARBONATE Sodium carbonate decahydrate 5968-11-6 Ethylene carbonate CarbohydrazideRelated product price
- MAGNESIUM CARBONATE
$235-18090 - Sodium carbonate
$14-4640 - Magnesium sulfate
$5-4104
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Hangzhou FandaChem Co.,Ltd.
Hefei TNJ Chemical Industry Co.,Ltd.
Hubei xin bonus chemical co. LTD
Chongqing Chemdad Co., Ltd
CONIER CHEM AND PHARMA LIMITED
Neostar United (Changzhou) Industrial Co., Ltd.





