|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
|
| 化学名: | 四酸化三鉛 | | 英語化学名: | lead oxide | | 别名: | granulated lead tetroxide;lead red granular;red lead oxide powder;LEAD OXIDE, RED, 99.99%;LEAD OXIDE, RED, POWDER, 1-2 MICRON, 99%;LeadOxide(RedLead);LEAD(II,IV) OXIDE (PB3O4);Lead, red | | CAS番号: | 1314-41-6 | | 分子式: | O4Pb3 | | 分子量: | 685.6 | | EINECS: | 215-235-6 | | カテゴリ情報: | Others ,Benzimidazoles;Inorganics;metal oxide | | Mol File: | 1314-41-6.mol | 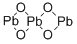 |
| 融点 | 500 °C | | 沸点 | 800°C | | 比重(密度) | 9,1 g/cm3 | | 蒸気圧 | 10 mm Hg ( 0 °C) | | 溶解性 | insoluble in H2O, ethanol; soluble in hotHCl | | 外見 | red powder | | 色 | Orange | | 水溶解度 | Soluble in hydrochloric acid, glacial acetic acid and nitric acid and hydrogen peroxide. Insoluble in water and alcohol. | | Merck | 14,5425 | | 暴露限界値 | ACGIH: TWA 0.05 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 | | Dielectric constant | 25.9(0.0℃) | | 安定性: | Stable. May react vigorously with reducing agents. | | InChIKey | XMFOQHDPRMAJNU-UHFFFAOYSA-N | | CAS データベース | 1314-41-6(CAS DataBase Reference) | | NISTの化学物質情報 | Lead(ii,iv) oxide(1314-41-6) | | EPAの化学物質情報 | Lead tetraoxide (1314-41-6) |
| | 四酸化三鉛 Usage And Synthesis |
| 外観 | 赤みの黄色~赤色, 粉末 | | 溶解性 | 水, エタノール, エーテルに不溶。 | | 解説 | Pb3O4(685.60).四酸化三鉛, 鉛丹,光明丹ともいう.また,実体はPbⅡ2PbⅣ O4であるので酸化鉛(Ⅳ)二鉛(Ⅱ)ともよばれる.鉛粉または粉状の酸化鉛(Ⅱ)を空気中で約450 ℃ に加熱すると得られる.赤色の無定形粉末.密度9.1 g cm-3.約550 ℃ に加熱すると分解し,酸素を放って酸化鉛(Ⅱ)となる.水に不溶であるが,硝酸を作用させると不溶性の二酸化鉛と硝酸鉛を生じる.赤色顔料,絵の具,ペイント,鉛ガラス,蓄電池の極板などに用いられる.有毒.森北出版「化学辞典(第2版) | | 用途 | ガラス添加剤 | | 用途 | 防錆塗料、鉛ガラス(管球用、光学用、クリスタル用他)、セラミック原料。 | | 説明 | Lead oxide, a range of products that is formed by the oxidation of Lead in the forms of liquid and solid. Lead oxides are basically an oxide’s family varying in color (grey/green, red, and yellow), in degree of oxidation (PbO, Pb3O4, PbO2) and in crystal structure (in forms of PbO, orthogonal and tetragonal).
Lead oxide is a term that can be either Lead monoxide or litharge Lead tetroxide or Red Lead or Gray or Black oxide which is a mixture of 30 percent metallic Lead and 70 percent Lead monoxide. Black Lead is made for specific use in Lead acid storage batteries manufacturing. Due to large use in the Lead acid battery industry, Lead monoxide is one of the most important compounds of Lead, based on volume. Due to its electrical and electronic properties, litharge is also used in various components for different types of use like capacitors, electro photographic plates, and Video tubes, even in ferromagnetic and ferroelectric materials. Their wide range of chemical and physical properties, Lead oxides have been know and used worldwide since before the ancient Romans. | | 化学的特性 | red to orange powder | | 使用 | Lead(II,IV) oxide is used to prepare colorless glass, faience glaze, porcelain painting flux, iron and steel coatings, rubber pigment and in glass cement. It is also used in gas and steam pipes, storage batteries, writing on glass and to make lead peroxide and matches. It is associated with linseed oil and used as a thick, long-lasting anti-corrosive paint. It gives better water resistant properties by replacing magnesium oxide. Further, it is used in the manufacture of lead glass and rustproof primer paints. In addition to this, it acts as a pigment for primer paints for iron objects. | | 使用 | Plasters and ointments; manufacture of colorless glass; glaze for faience; flux for porcelain painting, protective paint for iron and steel; oil-color for ship paints, varnishes; coloring rubber; cement for glass, gas and steam pipes; storage batteries; pencils for writing on glass; manufacture of lead peroxide, matches. | | 使用 | Red lead (Pb3O4) is a brilliant red-orange colored synthetic inorganic
pigment used mainly as a protective priming coat for steel work rather
than a coloring pigment in paints. The toxic nature of this pigment
restricts its use in modern coating systems. | | 調製方法 | Lead(IV) oxide, Pb2O, is obtained by action of chlorine on alkaline solutions of lead(II) oxide or acetate. The reaction is Pb(OH)3-+ ClO- → PbO2 + Cl- + OH- + H2O·PbO2 can also be produced on a lead or platinum anode by electrolysis in acidic solution. | | 定義 | Natural red oxide of lead. | | brand name | Hiroval;Wndomethasone. | | 世界保健機関(WHO) | Lead oxides and other lead salts were formerly available in
topical preparations which had soothing astringent properties. The toxicity of lead
salts by inhalation, ingestion and percutaneous absorption is now conclusively
established and the medicinal use of preparations containing lead salts is no
longer permitted in many countries. | | 燃焼性と爆発性 | Not classified | | 工業用途 | Lead oxide is used quite extensively in optical glass, electrical glass, and tableware. It increases the density and refractive index of glass. In addition, it can be cut more easily than other glasses and has superior brilliance, both of which make it good for cut glass.
Lead glasses may be formulated with a wide variety of electrical and acid-resisting characteristics; desirable properties, such as weather resistance, electrical resistivity, etc., will depend upon the total composition of the glass. |
|