|
ChemicalBook Optimization Suppliers |
| 名前: |
Joyochem Co.,Ltd Gold |
| 電話番号: |
531-82687558 13290333633 |
| 電子メール: |
sales@joyochem.com |
|
| 化学名: | プラスグレル | | 英語化学名: | Prasugrel | | 别名: | Prasugrel;2-[2-(Acetyloxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]-1-cyclopropyl-2-(2-fluorophenyl)ethanone;Ethanone, 2-(2-(acetyloxy)-6,7-dihydrothieno(3,2-C)pyridin-5(4H)-yl)-1-cyclopropyl-2-(2-fluorophenyl)-;Ly 640315;Ly640315;Ly-640315;Unii-34K66tbt99;5-(2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate | | CAS番号: | 150322-43-3 | | 分子式: | C20H20FNO3S | | 分子量: | 373.44 | | EINECS: | 801-962-1 | | カテゴリ情報: | LUVOX | | Mol File: | 150322-43-3.mol | 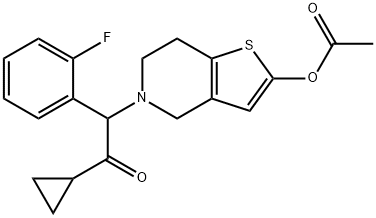 |
| 融点 | 120.0 to 124.0 °C | | 沸点 | 493.5±45.0 °C(Predicted) | | 比重(密度) | 1.347 | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: >5mg/mL (warmed at 60 °C) | | 酸解離定数(Pka) | 3.65±0.20(Predicted) | | 外見 | powder | | 色 | white to beige | | InChIKey | DTGLZDAWLRGWQN-UHFFFAOYSA-N | | CAS データベース | 150322-43-3(CAS DataBase Reference) |
| | プラスグレル Usage And Synthesis |
| 外観 | うすい黄色~黄色~黄赤色粉末~結晶 | | 説明 | Prasugrel is a third-generation thienopyridine that has been developed
and launched for the prevention of atherothrombotic events in patients
with ACS or following PCI. While the second-generation agent clopidogrel
was an improvement over the first-generation ticlopidine, which suffered
from gastrointestinal adverse effects and the risk of neutropenia with
prolonged use, its delayed onset of action and considerable interpatient
variability prompted the search for the next-generation thienopyridine.
The mechanism of action of these platelet inhibitors involves initial biological activation to a sulfhydryl metabolite that irreversibly binds to the
P2γ12 receptor on platelets via disulfide formation, thereby preventing platelet activation and aggregation by the endogenous agonist adenosine
diphosphate (ADP). The advantage of prasugrel over its predecessors is its
more efficient and consistent absorption and rapid conversion to its active
metabolite. Co-administration of thienopyridines with acetylsalicylic acid
(aspirin), an inhibitor of the synthesis of the platelet aggregation mediator
thromboxane A2, is an effective antiplatelet strategy and joins antagonists
of glycoprotein IIb/IIIa, which target the final step in platelet aggregation,
in the medical arsenal combating atherothrombotic events. | | Originator | Daiichi Sankyo (Japan) | | 使用 | Inhibits platelet aggregation
(platelet ADPP 2Y12 antagonist). | | 使用 | Prasugrel is a platelet inhibitor that reduces aggregation of platelets by being a P2Y12(ADP receptor) inhibitor. | | 定義 | ChEBI: 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate is a member of the class of thienopyridines that is 2-acetoxy-4,5,6,7-tetrahydrothieno[3,2-c]pyridine in which the amino hydrogen is replaced by a 2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl group. It is an acetate ester, a member of cyclopropanes, a ketone, a member of monofluorobenzenes, a tertiary amino compound and a thienopyridine. | | brand name | Effient | | 臨床応用 | Prasugrel is a platelet inhibitor developed by Daiichi Sankyo Co.
and is marketed in the United States in cooperation with Eli Lilly and Company for acute coronary syndromes planned for percutaneous
coronary intervention (PCI). Prasugrel was approved for
use in Europe in February 2009, and is currently available in the
UK. In the U.S. prasugrel is also approved for the reduction of
thrombotic cardiovascular events, including stent thrombosis, in
patients with acute coronary syndrome who are to be managed
with PCI. Prasugrel is a member of the thienopyridine class of
ADP receptor inhibitors, and irreversibly binds to P2Y12 receptors. | | 副作用 | In addition to the hemorrhagic side effect, other serious adverse events included AF, bradycardia, leucopenia, severe thrombocytopenia, angiodema, anemia, and abnormal hepatic function with hypertension, headache, back pain, dyspnea, nausea, dizziness, and diarrhea as less severe complaints. Prasugrel is contraindicated in patients with active pathological bleeding, such as peptic ulcers or intracranial hemorrhage, and in patients with a history of prior transient ischemic attack or stroke. In addition, in patients 75 years old, <60 kg, or likely to undergo urgent coronary artery bypass graft surgery, the risk may not outweigh the benefit. When possible, prasugrel treatment should be discontinued at least 7 days prior to any surgery. While warfarin and non-steroidal antiinflammatory drugs (NSAIDS) may increase the risk of bleeding with coadministration of prasugrel, no drug interactions are anticipated with concomitant use of drugs that are inducers or inhibitors of the cytochrome P450 enzymes. Prasugrel may also be administered with aspirin (75-325 mg per day), heparin, GP IIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including PPIs and H2 blockers. | | 合成 | The synthesis of prasugrel begins with the preparation of the
a-ketocyclopropane 102 which is prepared as summarized in the scheme. Conversion of 1-(bromomethyl)-2-fluorobenzene
(99) to the corresponding Grignard reagent through reaction with
magnesium followed by condensation with nitrile 100 resulted in
ketone 101 in 72% yield. Chlorination of ketone 101 with CuCl2 resulted
in the key prasugrel coupling component 102 in 92% yield.
The piperidine coupling partner was prepared by treating thiolactone
103 with TBDMSCl and triethylamine to give thiophene 104 in
91% yield. Treatment of piperidine 104 with a-chloroketone 102
resulted in enol silane 105 in 65% yield. Reaction of silylenol ether
105 with acetic anhydride in the presence of triethylamine and
catalytic DMAP resulted in the preparation of prasugrel (XVII) in
60% yield. 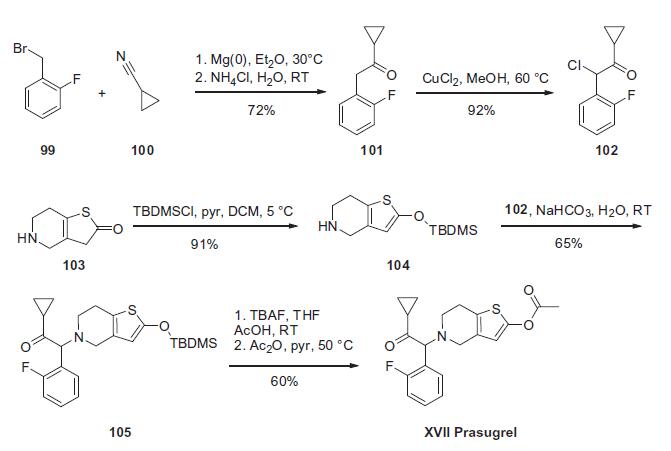
| | 薬物相互作用 | Potentially hazardous interactions with other drugs
Anticoagulants: enhanced anticoagulant effect with
coumarins and phenindione. | | 代謝 | Prasugrel is a prodrug and is rapidly metabolised in the
liver by various cytochrome P450 enzymes to an active
metabolite and inactive metabolites. The active metabolite
is further metabolised to 2 inactive compounds which are
excreted in the urine and faeces; about 68% of a dose is
excreted in urine and about 27% in faeces. | | 貯蔵 | Store at +4°C |
|