|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | カルシウム シアナミド | | 英語化学名: | Calcium cyanamide | | 别名: | CYANAMIDE, CALCIUM DERIVATIVE;CYANAMIDE CALCIUM SALT;CALCIUM CYANAMIDE;calcium cyanamide, tech.;CALCIUMCYANAMIDE,TECHNICAL;calcium cyanarnide;Cyanamide calcium derivative, Cyanamide calcium salt;Aero cyanamid granular | | CAS番号: | 156-62-7 | | 分子式: | CCaN2 | | 分子量: | 80.1 | | EINECS: | 205-861-8 | | カテゴリ情報: | Inorganics;Pharmaceutical Intermediates | | Mol File: | 156-62-7.mol | 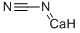 |
| 融点 | >300 °C(lit.) | | 比重(密度) | 2.29 | | 蒸気圧 | 0.51Pa at 20℃ | | 溶解性 | reacts with H2O | | 外見 | Powder | | 色 | Gray to dark gray | | 比重 | 2.29 | | 水溶解度 | insoluble H2O, but undergoes hydrolysis releasing acetylene and ammonia [HAW93] [MER06] | | Sensitive | Moisture Sensitive | | Merck | 14,1662 | | BRN | 4124391 | | InChIKey | QFSRQFUIHVTIDL-UHFFFAOYSA-N | | LogP | -0.72 at 20℃ | | CAS データベース | 156-62-7(CAS DataBase Reference) | | EPAの化学物質情報 | Calcium cyanamide (156-62-7) |
| | カルシウム シアナミド Usage And Synthesis |
| 外観 | うすい灰色~褐色~黒色粉末~結晶 | | 解説 | カルシウムシアナミド,CaCN2(80.10).炭化カルシウムCaC2を N2 中で強熱すると得られる.この反応は,空気中の窒素固定反応の最初の例である.純粋なものは無色の結晶.六方晶系で,ひずんだNaCl型の格子に,Ca2+ と直線形の [N-C-N]2- とが配列されている.N-C 1.22 Å.融点約1340 ℃.1150~1200 ℃ で昇華する.密度2.29 g cm-3.冷水と反応してNH3とCaCO3になり,熱水と反応してジシアンジアミド (NCNH2)2 になる.強アルカリ水溶液と反応して尿素を生じる.石灰窒素(灰色の粉末)は,CaCN2とCの混合物である.窒素肥料として,基肥に用いられる(土中で水分と反応して,まずジシアンジアミドとなり,数週間でさらに尿素と炭酸アンモニウムとになり,窒素肥料としてはたらく).しかし,このジシアンジアミドは植物に有害であり,速効が求められる追肥などには不適当である.一方,このジシアンジアミドの毒性を利用して,除草,殺菌,消毒などにも用いられる.そのほか,ジシアンジアミドやメラミンの合成原料,鋼の焼入れなどに用いられる. | | 用途 | 肥料、有機合成原料 | | 効能 | 抗酒癖薬, アルコール増感薬 | | 説明 | Calcium cyanamide is a blackish-gray, shinycrystalline material or powder. Molecular weight=80.11;Specific gravity (H2O:1)=2.29; Freezing/Melting point 51340℃ (sublimes .1500℃). Hazard Identification (basedon NFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 1. Insoluble in water; reaction. | | 化学的特性 | Calcium cyanamide is a blackish-gray, shiny crystalline material or powder.
 | | 物理的性質 | Pure product is a colorless, hexagonal crystal or white powder. Commercial grade material may be grayish-black powder or lump (the color is due to presence of calcium carbide and other impurities); density 2.29 g/cm3; melts around 1,340°C; sublimes around 1,150 to 1,200°C on rapid heating; reacts with water. | | 使用 | Manufacture of calcium cyanide and
dicyandiamide; formerly used as a defoliant and
herbicide | | 使用 | Calcium cyanamide has
its major use as a fertilizer. However, it has a number of other
uses, such as a herbicide and a defoliant for cotton plants. It is
finding increasing use as a chemical intermediate. For example,
it is being used to produce dicyandiamide, which in turn can be polymerized to form the widely used monomer,
melamine. The conversion to calcium cyanide and hence
into a variety of other uses is also important commercially. | | 使用 | Calcium Cyanamide is used as a fertilizer, herbicide, insecticide, a steel-making additive and an ore processing material. It can also be used to make thiourea, guanidine and ferrocyanides. manufacture of calcium cyanide, melamine, dicyandiamide. | | 製造方法 | Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1,000°C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.
CaC2 + N2 → CaCN2 + C (ΔHƒ°= –69.0 kcal/mol at 25°C) | | 調製方法 | Calcium cyanamide was first produced commercially around
1900 as a fertilizer. The process of making calcium cyanamide
involves three raw materials—coke, coal, and limestone—
plus nitrogen. The limestone (calcium carbonate) is
burned with coal to produce calcium oxide. The calcium
oxide is then allowed to react with amorphous carbon in the
furnace at 2000°C with the formation of calcium carbide
(CaC2). Finely powdered calcium carbide is heated to
1000°C in an electric furnace into which pure nitrogen
is passed. It is then removed and uncombined calcium
carbide removed by leaching. | | 定義 | calcium cyanamide: A colourlesssolid, CaCN2, which sublimes at1300°C. It is prepared by heating calciumdicarbide at 800°C in a streamof nitrogen:
CaC2(s) + N2(g) → CaCN2(s) + C(s)
The reaction has been used as amethod of fixing nitrogen in countriesin which cheap electricity isavailable to make the calcium dicarbide(the cyanamide process). Calciumcyanamide can be used as afertilizer because it reacts with waterto give ammonia and calcium carbonate:
CaCN2(s) + 3H2O(l) → CaCO3(s) +2NH3(g)
It is also used in the production ofmelamine, urea, and certain cyanidesalts. | | 定義 | ChEBI: The calcium salt of cyanamide, formed when calcium carbide reacts with nitrogen | | 工業製造 | 炭酸カルシウムまたは酸化カルシウムに、600~850℃でアンモニアと一酸化炭素とを反応させると得られる。工業的には、細粒ないし粉末状の炭化カルシウムを、窒素気流中で約1000℃で加熱する方法がとられる。 CaC2+N2―→CaCN2+C この場合は黒鉛状の微粉末炭素を副生するので、生成物は黒色を呈する。この混合物を石灰窒素という。純粋なものは無色の固体。水に溶け徐々に加水分解されてアンモニアと炭酸カルシウムとになる。 CaCN2+3H2O―→2NH3+CaCO3 加熱によりこの分解は促進される。強アルカリ水溶液と反応して尿素を生成し、高温では炭素と作用してシアン化カルシウムを生ずる。 | | 一般的な説明 | A colorless to gray, odorless solid. May cause illness from ingestion. May irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers. | | 空気と水の反応 | Depending on the calcium carbide content, the cyanamide reacts with water (moisture from air or soil) to produce acetylene and hydrated calcium oxide or calcium hydroxide. Absorption of water during handling or storage of technical calcium cyanamide may cause explosion [Pieri, M. Chem. Abs. 46, 8335 1952]. | | 反応プロフィール | When hydrated CALCIUM CARBIDE generates salts of calcium that are basic and are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. | | 危険性 | Fire risk with moisture or combined with
calcium carbide. Skin, eye, and upper respiratory
tract irritant. Questionable carcinogen. | | 健康ハザード | Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. | | 火災危険 | Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard. | | 燃焼性と爆発性 | Non flammable | | 农业用途 | Calcium cyanamide (CaCN2) is a dark colored,
granulated material containing around 21 % nitrogen. Its
dark color is due to the presence of calcium carbide.
Calcium cyanamide is produced by heating a mixture
of limestone with coal in a nitrogen atmosphere.
Generally, the process is carried out in three steps. In the
first step, calcium carbonate (limestone) is decomposed
at about 1100°C.
In the second step, calcium oxide (CaO) and coke (or
coal) are heated in an electric furnace to produce calcium
carbide.
The final step involves heating the powdered calcium
carbide at about 1100°C with pure nitrogen (produced by
liquefaction of air and fractional distillation) to produce
calcium cyanamide.
The fertilizer-grade calcium cyanamide contains 21 %
nitrogen, 11 % calcium, 11 % free carbon, 5% oil, 2 to
4% water and oxides of aluminum, iron and silicon. In
the presence of moisture and air, calcium
dicyandiamide (a poisonous compound) is formed. It
distinctly leaves alkalinity in the soil equivalent to 1.3 kg
calcium carbonate (CaCO3) per 0.45 kg of nitrogen
applied. At pH 7 or below, calcium dicyandiamide is
converted into urea and lime within one week of its being
in the soil.
When dry, calcium cyanamide is dusty but it is
generally used as granules. It is poisonous, irritating to
the skin and used as a pesticide, fertilizer and defoliant in
cotton. It is as good a fertilizer as sodium nitrate or
ammonium sulphate, but not as fast acting.
Calcium cyanamide is an excellent weed killer,
especially for tobacco plants, when applied 2 to 3 weeks
before sowing. It is also used for producing melamine,
urea and certain cyanide salts. | | 安全性プロファイル | Poison by ingestion,
inhalation, sh contact, intravenous, and
intraperitoneal routes. Moderately toxic to
humans by ingestion. Questionable
carcinogen with experimental tumorigenic
data. Mutation data reported. The fatal dose,
by ingestion, is probably around 20 to 30 g
for an adult. It does not have a cyanide
effect. Calcium cyanamide is not believed to
have a cumulative action. Flammable.
Reaction with water forms the explosive
acetylene gas. When heated to
decomposition it emits toxic fumes of NOx
and CN-. See also CALCIUM
COMPOUNDS, AMIDES, and
CYANIDE | | 職業ばく露 | Calcium cyanamide is used in agriculture as a fertilizer, herbicide; defoliant for cotton plants;
and pesticide. It is also used in the manufacture of dicyandiamide and calcium cyanide as a desulfurizer in the iron
and steel industry; and in steel hardening. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | 発がん性 | Calcium cyanamide was weakly mutagenic
in Salmonella typhimurium strain TA1535 and
nonmutagenic in strain TA100. | | 貯蔵 | Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with calciumcyanamide you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from moisture. A regulated, markedarea should be established where this chemical is handled,used, or stored in compliance with OSHA Standard1910.1045. | | 輸送方法 | UN1403 Calcium cyanamide with .1% calcium
carbide, Hazard Class: 4.3; Labels: 4.3-Dangerous when
wet material | | 不和合性 | Commercial grades of calcium cyanamide may contain calcium carbide; contact with any form
of moisture solutions may cause decomposition, liberating
explosive acetylene gas and ammonia. Incompatible with
oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May
polymerize in water or alkaline solutions to dicyanamide.
Contact with all solvents tested also causes decomposition |
|