|
ChemicalBook Optimization Suppliers |
|
| 融点 | 133-137 °C (lit.) | | 沸点 | 294.5 °C/100 mmHg (lit.) | | 比重(密度) | 1.21 | | 蒸気圧 | 1 mm Hg ( 183 °C) | | 屈折率 | 1.422 | | 闪点 | 220 °C | | 貯蔵温度 | Store below +30°C. | | 溶解性 | ethanol: 100 mg/mL | | 外見 | Powder or Granules | | 酸解離定数(Pka) | 4.59, 5.59(at 25℃) | | 色 | White to off-white | | 臭い (Odor) | monoclinic prismatic tablets, wh. powd., fatty acid odor | | 水溶解度 | 1 g/L (20 ºC) | | Merck | 14,8415 | | BRN | 1210591 | | 安定性: | Stable. Combustible. Incompatible with strong oxidizing agents, bases, reducing agents. | | InChIKey | CXMXRPHRNRROMY-UHFFFAOYSA-N | | LogP | 1.5 at 23℃ | | CAS データベース | 111-20-6(CAS DataBase Reference) | | NISTの化学物質情報 | Decanedioic acid(111-20-6) | | EPAの化学物質情報 | Sebacic acid (111-20-6) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 26-36-24/25 | | WGK Germany | 1 | | RTECS 番号 | VS0875000 | | TSCA | Yes | | HSコード | 29171310 | | 毒性 | LD50 orally in Rabbit: 3400 - 14500 mg/kg LD50 dermal Rat > 2000 mg/kg |
| | セバシン酸 Usage And Synthesis |
| 外観 | 白色~ほとんど白色, 小粒 | | 定義 | 本品は、次の化学式で表されるジカルボン酸である。 | | 溶解性 | 水に難溶, エタノール及びジエチルエーテルに可溶。エタノールに溶け、水に溶けにくい。 | | 解説 | セバシン酸無色の葉状結晶.融点134.5 ℃,沸点294.5 ℃(13.3 kPa),232 ℃(1.33 kPa).d420"1.422.エタノール,エーテルに易溶,水に難溶.アルキド樹脂,ポリアミドの製造原料,また,ろうそくや香料の原料にも用いられる. | | 用途 | 可塑剤、アルキド樹脂、ポリアミド、潤滑油、ペイント、ロウソク、香料の原料。 | | 製造 | セバシン酸,トウゴマの種子から得られるヒマシ油が主原料の直鎖ジカルボン酸です。ひまし油あるいはリシノール酸を水酸化アルカリと乾留するか,アジピン酸エチルカリウムを電解すると得られる. | | 化粧品の成分用途 | pH調整剤 | | 説明 | Sebacic acid is a dicarboxylic acid with structure (HOOC)(CH2)8(COOH), and is naturally occurring.
In its pure state it is a white flake or powdered crystal. The product is described as non-hazardous, though in its powdered form it can be prone to flash ignition (a typical risk in handling fine organic powders).
Sebaceus is Latin for tallow candle, sebum (tallow) is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil, with the vast majority of world production occurring in China which annually exports over 20,000 metric tonnes, representing over 90 % of global trade of the product.
In the industrial setting, sebacic acid and its homologues such as azelaic acid can be used in plasticizers, lubricants, hydraulic fluids, cosmetics, candles, etc. Sebacic acid is also used as an intermediate for aromatics, antiseptics, and painting materials. | | 化学的特性 | White flaky crystals. Slightly soluble in water, soluble in alcohol and ether. | | 使用 | Sebacic Acid is a urinary metabolite that has been identified as an anti-fatigue biomarker. | | 使用 | Decanedioic acid was named by Thenard LJ (1802) from the Latin sebaceus(tallow candle) or sebum (tallow) in reference to its use in the manufacture of candles. Thenard LJ isolated this compound from distillation products of beef tallow. In 1954, it was reported that it was produced in excess of 10,000 tons annually by alkali fission of castor oil. Sebacic acid and its derivatives, as azelaic acid, have a variety of industrial uses as plasticizers, lubricants, diffusion pump oils, cosmetics, candles, etc. It is also used in the synthesis of polyamide, as nylon, and of alkyd resins. An isomer, isosebacic acid, has several applications in the manufacture of vinyl resin plasticizers, extrusion plastics, adhesives, ester lubricants, polyesters, polyurethane resins and synthetic rubber. | | 定義 | ChEBI: Sebacic acid is an alpha,omega-dicarboxylic acid that is the 1,8-dicarboxy derivative of octane. It has a role as a human metabolite and a plant metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of a sebacate(2-) and a sebacate. It derives from a hydride of a decane. | | 製造方法 | Sebacic acid is normally made from castor oil, which is essentially glycerol
triricinoleate. The castor oil is heated with sodium hydroxide at about 250??e.
This treatment results in saponification of the castor oil to ricinoleic acid
which is then cleaved to give 2-octanol and sebacic acid:
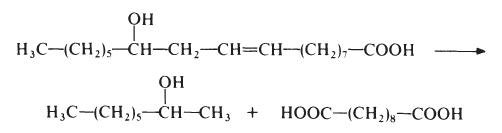
This process results in low yields of sebacic acid (about 50% based on the
castor oil) but, nevertheless, other routes have not proved competitive.
Sebacic acid is a colourless crystalline solid, m.p. 134??. | | 一般的な説明 | White granular powder. Melting point 153°F. Slightly soluble in water. Sublimes slowly at 750 mm Hg when heated to melting point. | | 空気と水の反応 | Insoluble in water. | | 反応プロフィール | Sebacic acid reacts exothermically to neutralize bases, both organic and inorganic. May react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb enough water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions. | | 火災危険 | Flash point data for Sebacic acid are not available. Sebacic acid is probably combustible. | | 燃焼性と爆発性 | Not classified | | 純化方法 | Purify sebacic acid via the disodium salt which, after crystallisation from boiling water (charcoal), is again converted to the free acid. The free acid is crystallised repeatedly from hot distilled water or from Me2CO/pet ether and dried under vacuum. [Beilstein 2 IV 2078.] |
|