|
ChemicalBook Optimization Suppliers |
|
| | アルミノン Usage And Synthesis |
| 外観 | 暗赤褐色, 結晶〜粉末 | | 溶解性 | 水に易溶。エタノール, エーテルに殆ど不溶。水に溶けやすく、エタノール及びジエチルエーテルにほとんど溶けない。水に溶けやすく、エタノール及びエーテルにほとんど溶けない。 | | 解説 | アウリントリカルボル酸アンモニウム(aurintricarboxylic acid ammonium salt)ともいう.濃硫酸中でサリチル酸とホルマリンを結合させて得られるアウリントリカルボン酸をアンモニアでアンモニウム塩にすると得られる.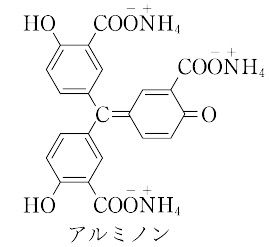 "赤褐色の粉末.中性あるいは弱酸性溶液では黄褐色(pH = 4で吸光度最大),アルカリ性で淡黄色.Al3+,Cr3+,Fe3+ などと有色の錯体をつくり,比色分析用試薬として用いられる.たとえば,微量の Al3+ イオンと酢酸緩衝溶液中で反応し,深赤色となる.[CAS 569-58-4] "赤褐色の粉末.中性あるいは弱酸性溶液では黄褐色(pH = 4で吸光度最大),アルカリ性で淡黄色.Al3+,Cr3+,Fe3+ などと有色の錯体をつくり,比色分析用試薬として用いられる.たとえば,微量の Al3+ イオンと酢酸緩衝溶液中で反応し,深赤色となる.[CAS 569-58-4]
森北出版「化学辞典(第2版) | | 用途 | 分析用試薬 : 1)微量のアルミニウムの検出(検出限界 2.5~5μg)および比色定量用試薬として用いる。pH5.3で発色させ、波長 525mμで測定する。2)AlF6(-3)の生成による退色反応を利用してフッ化物を定量するのに用いられる。 | | 化学的特性 | dark red crystalline powder | | 使用 | It is a potent inhibitor of ribonuclease and topoisomerase II by preventing the binding of the nucleic acid to the enzyme. It stimulates tyrosine phosphorylation processes including the Jak2/STAT5 pathway in NB2 lymphoma cells, ErbB4 in neuroblastoma cells, and MAP kinases, Shc proteins, phosphatidylinositide 3-kinase and phospholipase C in PC12 cells. Aurintricarboxylic acid inhibits apoptosis in many cell types. | | 使用 | Aluminon can used as a reagent for aluminum in solution. It is also Forms brilliantly colored lakes with aluminum, chromium, iron, beryllium. Generally used for the detection and colorimetric estimation of aluminum in water, foods, tissues. Cf. Scherrer, Mogerman, J. Res. Natl. Bur. Stand. 21, 105 (1938). | | 定義 | ChEBI: An ammonium salt that is the ammonium salt of 3,3'-[(3-carboxy-4-oxocyclohexa-2,5-dien-1-ylidene)methylene]bis(6-hydroxybenzoic acid). A dye commonly used to detect the presence of the aluminium ion in an aqueous solution. | | Biochem/physiol Actions | Aurintricarboxylic acid readily polymerizes in aqueous solution, forming a stable free radical that inhibits protein-nucleic acid interactions. It is a potent inhibitor of ribonuclease and topoisomerase II by preventing the binding of the nucleic acid to the enzyme. It stimulates tyrosine phosphorylation processes including the Jak2/STAT5 pathway in NB2 lymphoma cells, ErbB4 in neuroblastoma cells, and MAP kinases, Shc proteins, phosphatidylinositide 3-kinase and phospholipase C in PC12 cells. Aurintricarboxylic acid inhibits apoptosis in many cell types. Its neuroprotective effect, perhaps due to its ability to prevent down-regulation of Ca2+ impermeable GluR2 receptors or to its ability to inhibit calpain, a Ca2+-activated protease that is activated during apoptosis. | | 安全性プロファイル | Human reproductive effects by ingestion: changes in male fertility. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NH3 and NOx,. |
|