|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 1,2-ジブロモエタン-1,2-ジオン | | 英語化学名: | OXALYL BROMIDE | | 别名: | oxalyl bromide solution;OXALYL BROMO;BrCOCOBr;Ethanedioyl bromide;ethanedioyldibromide;Ethanedioyl-dibromide-;OXALYL BROMIDE, 2.0M SOLUTION IN DICHLOR OMETHANE;Dibromoethanedione | | CAS番号: | 15219-34-8 | | 分子式: | C2Br2O2 | | 分子量: | 215.83 | | EINECS: | 239-271-7 | | カテゴリ情報: | Others;Oxidation;Synthetic Reagents | | Mol File: | 15219-34-8.mol | 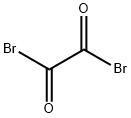 |
| 融点 | -19 °C (lit.) | | 沸点 | 16-17 °C/10 mmHg (lit.) | | 比重(密度) | 1.517 g/mL at 25 °C | | 屈折率 | n20/D 1.522(lit.) | | 闪点 | -19°C | | 貯蔵温度 | 2-8°C | | 溶解性 | Benzene (Slightly), Chloroform | | 外見 | Liquid | | 色 | Clear | | 由来生物 | goat | | BRN | 1744437 | | 安定性: | Moisture Sensitive | | InChI | InChI=1S/C2Br2O2/c3-1(5)2(4)6 | | InChIKey | JAZLVNXWYDFQFE-UHFFFAOYSA-N | | SMILES | C(Br)(=O)C(Br)=O | | CAS データベース | 15219-34-8 | | EPAの化学物質情報 | Ethanedioyl dibromide (15219-34-8) |
| | 1,2-ジブロモエタン-1,2-ジオン Usage And Synthesis |
| 化学的特性 | clear yellow to yellow-green liquid | | 使用 | Used in synthetic applications of carbon-substituted iminium salts
Reactant for:
Synthesis of mutasynthons added to cultures of A. pretiosum for mutasynthetic generation of ansamitocin derivatives
Oxalic acid formation from hydroxyl radical substitutions
Cyclization to produce CRF1 receptor antagonists
Preparation, optical and electrochemical studies of thiophene end capped olig(2,3-alkylthieno[3,4-b]pyrazine)
Asymmetrical synthesis of glycosyl chlorides and bromides | | 使用 | Leptin from rat has been used:
- to check the effect of intranasal nerve growth factor (NGF) on NGF activity in the spinal cord of Sprague-Dawley rats
- for validation of labelling for leptin-bearing cells of Sprague-Dawley rats
- to study the effect of leptin on STAT3 (signal transducer and activator of transcription 3) phosphorylation in neural population of Sprague-Dawley rats
- to study leptin response towards chemoreflex in nucleus of the solitary tract
- in rats, to check the effect of association between leptin and neuronal nitric oxide pathway on penicillin-induced epileptiform activity
| | reaction suitability | reagent type: oxidant | | Biochem/physiol Actions | Leptin is a hormone produced primarily in adipocytes, although leptin mRNA has also been identified in placenta and fetal tissues, gastric tissue and liver. Its primary site of action appears to be on neurons in the hypothalamus that are involved in regulating energy balance, appetite, and body weight. Leptin increases the production of nitric oxide in endothelial cells and stimulates angiogenesis in vitro and in vivo.Human and mouse leptin share ~84% sequence identity. |
|