|
ChemicalBook Optimization Suppliers |
|
| 化学名: | イリノテカン | | 英語化学名: | Irinotecan | | 别名: | 1,4'-Bipiperidine-1'-carboxylic acid (S)-4,11-diethyl-3,4,12,14- tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester;DQ-2805;(4S)-4,11-Diethyl-4-hydroxy-9-[[(4-piperidinopiperidino)carbonyl]oxy]-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione;SN-38-11;[1,4'-Bipiperidine]-1'-carboxylic acid, 4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, (S)-;1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline, [1,4'-bipiperidine]-1'-carboxylic acid deriv.;Irinotecan base;(4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl-[1,4'-bipiperidine]-1'-carboxylic acid | | CAS番号: | 97682-44-5 | | 分子式: | C33H38N4O6 | | 分子量: | 586.68 | | EINECS: | 691-567-9 | | カテゴリ情報: | Camptothecin series;APIs;Natural Anti-cancer Medical Materials and It's Derivatives;API's;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;API;Anti cancer;Inhibitors;Camptosar, Campto;Anti-cancer&immunity | | Mol File: | 97682-44-5.mol | 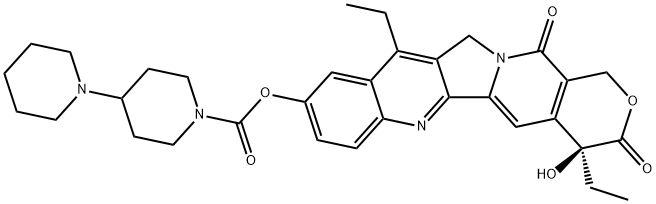 |
| 融点 | 222-223° | | 沸点 | 873.4±65.0 °C(Predicted) | | 比重(密度) | 1.40±0.1 g/cm3(Predicted) | | 貯蔵温度 | Sealed in dry,2-8°C | | 溶解性 | Acetonitrile (Slightly, Heated, Sonicated), DMSO (Slightly), Methanol (Slightly) | | 外見 | Solid | | 酸解離定数(Pka) | 11.20±0.20(Predicted) | | 色 | White to Brown | | CAS データベース | 97682-44-5(CAS DataBase Reference) |
| | イリノテカン Usage And Synthesis |
| 効能 | 抗悪性腫瘍薬, トポイソメラーゼI阻害薬 | | 使用 | (+)-Irinotecan is a Topo I inhibitor. | | 使用 | Irinotecan is a topoisomerase I inhibitor for LoVo cells and HT-29 cells with IC50 of 15.8 μM and 5.17 μM, respectively | | 定義 | ChEBI: A member of the class of pyranoindolizinoquinolines that is the carbamate ester obtained by formal condensation of the carboxy group of [1,4'-bipiperidine]-1'-carboxylic acid with the phenolic hydroxy group of (4S)-4,11-diethyl-4,9-dihydro
y-1H-pyrano[3',4':6,7]indolizino[1,2- hydrochloride]quinoline-3,14-dione. Used (in the form of its hydrochloride salt trihydrate) in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcino
a of the pancreas after disease progression following gemcitabine-based therapy. It is converted via hydrolysis of the carbamate linkage to its active metabolite, SN-38, which is ~1000 times more active. | | brand name | Camptosar (Pharmacia &Upjohn)
. | | 生物活性 | irinotecan (cpt-11), a prodrug for treating metastatic colorectal cancer, is a topoisomerase i inhibitor for lovo cells and ht-29 cells with ic50 of 15.8 μm and 5.17 μm, respectively [1].in vivo, irinotecan is converted to sn-38, its most active metabolite, by carboxylesterase converting enzyme (cce) [2]. | | in vitro | irinotecan induced similar amounts of cleavable complexes in lovocells and ht-29 cell lines with the ic50 of 15.8 μm and 5.17 μm, respectively [1].after addition of 157 mm irinotecan to plasma, sn-38 concentration showed linear increase during the first 60-min period, followed by a plateau.in the first 60 min, mean and standard deviation of the conversion rate were 515.9 ± 50.1 pmol/ml/h (n = 69), with a coefficient of variation of 0.097 [2]. irinotecan (cpt-11) was significantly more active in sclc than in nsclccelllines (p = 0.0036). ce activity appeared to be associated with higher sensitivity to cpt-11 in human lung cancercelllines and may partly explain the difference in the in vitro sensitivity to cpt-11 between sclc and nsclccells [3].in vitro, the sensitivity to cpt-11 and sn-38 was highest in ls174t and colo 320cells, intermediate in sw1398cellsand lowest in colo 205 and widr cells. the activity of sn-38 was 130 to 570 times than cpt-11[4]. | | in vivo | in colo 320 xenografts, irinotecan induced a maximum growth inhibition of 92% [4].a single dose of irinotecan significantly increased amounts of topoisomerase i covalently bound to dna in stomach, duodenum, colon and liver. concomitantly, the irinotecan-treated group exihibited significantly higher amounts of dna strand breaks in colon mucosa cells compared to the control group [5]. | | 参考文献 | [1]. tobin p, clarke s, seale j p, et al. the in vitro metabolism of irinotecan (cpt‐11) by carboxylesterase and β‐glucuronidase in human colorectal tumours[j]. british journal of clinical pharmacology, 2006, 62(1): 122-129.
[2]. shingyoji m, takiguchi y, watanabe‐uruma r, et al. in vitro conversion of irinotecan to sn‐38 in human plasma[j]. cancer science, 2004, 95(6): 537-540.
[3]. van ark-otte j, kedde m a, van der vijgh w j, et al. determinants of cpt-11 and sn-38 activities in human lung cancer cells[j]. british journal of cancer, 1998, 77(12): 2171.
[4]. jansen w j m, zwart b, hulscher s t m, et al. cpt-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants[j]. international journal of cancer, 1997, 70(3): 335-340.
[5]. na y s, jung k a, kim s m, et al. the histone deacetylase inhibitor pxd101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models[j]. cancer chemotherapy and pharmacology, 2011, 68(2): 389-398. |
|