|
ChemicalBook Optimization Suppliers |
| 名前: |
BEST-REAGENT |
| 電話番号: |
400-1166-196 18981987031 |
| 電子メール: |
cdhxsj@163.com |
|
| 化学名: | フェンチオン | | 英語化学名: | Fenthion | | 别名: | LEBAYCID;LEBAYCID(R);FENCHEM;FENTHION;FASTER;ENTEX(R);ENT 25540;BEILIULIN | | CAS番号: | 55-38-9 | | 分子式: | C10H15O3PS2 | | 分子量: | 278.33 | | EINECS: | 200-231-9 | | カテゴリ情報: | NITROPRESS;INSECTICIDE | | Mol File: | 55-38-9.mol | 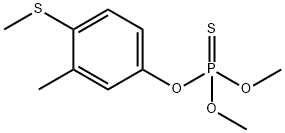 |
| 融点 | 7.5°C | | 沸点 | 87°C (0.01 mmHg) | | 比重(密度) | 1.25 | | 蒸気圧 | 7.4 x 10-4 Pa (20 °C) | | 屈折率 | nD20 1.5698 | | 闪点 | >100 °C | | 貯蔵温度 | 2-8°C | | 溶解性 | Chloroform (Sparingly), DMSO (Sparingly) | | 外見 | liquid | | 水溶解度 | 0.0055 g/100 mL | | Merck | 13,4030 | | BRN | 1974129 | | 暴露限界値 | OSHA PEL: TWA 0.2 mg/m3; ACGIH TLV: TWA 0.2 mg/m3 | | 安定性: | Stable. Incompatible with strong oxidizing agents. | | CAS データベース | 55-38-9(CAS DataBase Reference) | | NISTの化学物質情報 | Fenthion(55-38-9) | | EPAの化学物質情報 | Fenthion (55-38-9) |
| | フェンチオン Usage And Synthesis |
| 溶解性 | 水に不溶 (6.3×10^(-4)g/100ml, 20℃)。脂肪族炭化水素以外の殆どの有機溶剤に可溶。水4.2mg/l(20℃)。ジクロロメタン, トルエン,イソプロパノール>1,000, ヘキサン30~100(共にg/l 20℃)。アセトンに溶け、水にほとんど溶けない。 | | 用途 | MPP試験用標準品。 | | 用途 | 農薬 | | 効能 | 殺虫薬 (獣医薬), アセチルコリンエステラーゼ阻害薬 | | 農薬用途 | 殺虫剤 | | 説明 | Fenthion is a colorless oily liquid (technical grade, brown oily
liquid with a mercaptan-like odor). | | 化学的特性 | Pure fenthion is a colorless liquid. Technical fenthion is a yellow or brown oily liquid with a weak garlic odor. It is insoluble or very sparingly soluble in water, but soluble in all organic solvents, alcohols, ethers, esters, halogenated aromatics, and petroleum ethers. It is grouped by the US EPA under RUP and hence requires handling by qualifi ed, certifi ed, and trained workers. Fenthion is used for the control of sucking and biting pests, i.e., fruit fl ies, stem borers, mosquitoes, and cereal bugs. In mosquitoes, it is toxic to both the adult and immature forms (larvae). The formulations of fenthion include dust, emulsifi able concentrate, granular, liquid concentrate, spray concentrate, ULV, and wettable powder. | | 化学的特性 | yellow brown oily liquid | | 化学的特性 | Fenthion is a colorless liquid (when pure)
with a weak, garlic-like odor. The technical grade is a
yellow-to-brown oil. | | 使用 | Insecticide for control of fruit ?ies, leaf hoppers and cereal bugs. Fenthion is also used as an acaracide. | | 使用 | Insecticide; acaricide. | | 使用 | antihypertensive | | 使用 | Fenthion is an organothiophosphate used as an insecticide. Fenthion is also a potent acaricide. | | 定義 | ChEBI: An organic thiophosphate that is O,O-dimethyl hydrogen phosphorothioate in which the hydrogen atom of the hydroxy group is replaced by a 3-methyl-4-(methylsulfanyl)phenyl group. It exhibits acaricidal and insecticidal
activities. | | 一般的な説明 | Yellow to tan oily liquid with a slight odor of garlic. | | 反応プロフィール | Fenthion may react with strong reducing agents such as hydrides to generate highly toxic and flammable phosphine gas. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. | | 危険性 | Toxic by ingestion, inhalation, and skin
absorption; use may be restricted, cholinesterase
inhibitor. Toxic by skin absorption; questionable
carcinogen. | | 健康ハザード | Fenthion is moderately toxic to mammals, and highly toxic to birds. Exposures to fenthion cause poisoning with symptoms among occupational workers as observed with organophosphate pesticide-induced toxicity. These include, but are not limited to, numbness, tingling sensations, incoordination, headache, dizziness, tremor, nausea, abdominal cramps, sweating, blurred vision, diffi culty breathing or respiratory depression, and slow heart beat. Very high doses may result in unconsciousness, incontinence, and convulsions or fatality. Reports have indicated that exposures to fenthion cause adverse effects on the central and peripheral nervous systems, and the heart of exposed workers. | | 火災危険 | Fenthion is probably combustible. | | 农业用途 | Insecticide, Larvicide, Veterinary medicine: Not registered for use in the U.S. Not approved for
use in EU countries. Used in 20 countries including
Australia. There are 23 global suppliers. | | 製品名 | B 29493®; BACID®; BAY 29493®;
BAYCID®; BAYER 29493®; BAYER 9007®; BAYER
S-1752®; BAYTEX®; ENTEX®; LEBAYCID®;
MPP®; MPP (pesticide) OMS 2®; PHENTHION®;
PILARTEX®; QUELETOX®; S 1752®; SPOTTON®;
SULFIDOPHOS®; TALODEX®; TIGUVON® | | 職業ばく露 | A potential danger to those involved
in the manufacture, formulation, or application of this agricultural chemical and pesticide; insecticide | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Ifexposure and/or symptoms have occurred, the person shouldbe under medical observation for several days, as somesymptoms may be delayed. | | 環境運命予測 | Biological. From the first-order biotic and abiotic rate constants of fenthion in estuarine water and sediment/water systems, the estimated biodegradation half-lives were 22.4 and 3.9–14.5 days, respectively (Walker et al., 1988).
Surface Water. In estuarine water, the half-life of fenthion was 4.6 days (Lacorte et al., 1995).
Plant. In plants, fenthion oxidizes to the mesulfenfos and sulfone which further degrades to the sulfone phosphate before undergoing hydrolysis (Hartley and Kidd, 1987).
Photolytic. Fenthion was oxidized to the corresponding sulfoxide and trace amounts (<5% yield) of sulfone when sorbed on soil and exposed to sunlight. The photosensitized oxidation was probably due to the presence of singlet oxygen. The degradation rate was higher in soils containing the lowest organic carbon (Gohre and Miller, 1986).
Chemical/Physical. Stable at temperatures below 210°C (Worthing and Hance, 1991). Emits very toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987; Lewis, 1990). | | 代謝経路 | The major route of fenthion metabolism is by oxidation to the sulfoxide
which has a higher insecticidal activity than the parent compound. The
subsequent oxidation of the sulfoxide to fenthion sulfone, the biological
activity of which is considerably lower, is slow in plants but of major
significance in animals. An additional route of bioactivation is through
oxidative desulfuration to form fenoxon and, in all, five oxidative
metabolites (fenthion sulfoxide, fenthon sulfone, fenoxon, fenoxon
sulfoxide and fenoxon sulfone) have been detected in most biological
systems. Hydrolysis, in contrast to most insecticidal phosphorothioates,
appears to proceed via direct hydrolysis of fenthion rather than its oxon.
The phenolic leaving group (3-methyl-4-thiomethylphenol) is identical to
that of fenamiphos and although no studies have described the fate of the
phenolic moiety for fenthion, it is likely that it will be metabolised by
similar routes to those described for fenamiphos. | | 貯蔵 | : Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers, alkalies and alkalinepesticides. A regulated, marked area should be establishedwhere this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045. | | 輸送方法 | UN3018 Organophosphorus pesticides, liquid,
toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required. | | Toxicity evaluation | Acute oral LD50 for rats: ca. 250 mg /kg | | Degradation | Fenthion is stable in light, stable to acid and moderately stable to alkaline
conditions. The DT50s at pH 4, 7 and 9 were 223, 151 and 151 days
respectively (PM).
The effect of the cuticular waxes of fruit upon the photodegradation of
fenthion was studied by Cabras et al. (1997). Waxes were extracted with
acetone and the fenthion added to the solution. The wax plus fenthion
was applied as a film which was irradiated by natural sunlight. The
analysis of fenthion and its metabolites was by GLC-FID (Cabras and
Plumitallo, 1991). The potential metabolites synthesised were fenthion
sulfoxide (2), fenthion sulfone (3), fenoxon (4), fenoxon sulfoxide (5) and
fenoxon sulfone (6). Waxes from different fruits affected the decay rate,
with wax from oranges and nectarines increasing the rate of decomposition
but cuticular wax from olives retarding the rate. In all cases the
products of photodegradation were fentluon sulfoxide (2), which
steadily increased with time, and fenthion sulfone (3), which remained at
a low but detectable level throughout the experiment. Minelli et al. (1996)
exposed fenthion in glass jars to natural sunlight and analysed the photodegradation
products by GLC-FID. The major product was fenthion
sulfoxide (2) which very slowly degraded to the sulfone (3). This in turn
was even more slowly photo-oxidised to fenoxon sulfone (6). The routes
for the photodegradation of fenthion are shown in Scheme 1. | | 不和合性 | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, alkaline insecticides.
Organothiophosphates are susceptible to formation of
highly toxic and flammable phosphine gas in the presence
of strong reducing agents such as hydrideds and active
metals. Partial oxidation by oxidizing agents may result in
the release of toxic phosphorus oxides. | | 廃棄物の処理 | Hydrolysis and landfill for
small quantities; incineration with flue gas scrubbing for
large amounts. In accordance with 40CFR165, follow
recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following
package label directions or by contacting your local or federal environmental control agency, or by contacting your
regional EPA office |
|