|
|
| | meterelin Basic information |
| Product Name: | meterelin | | Synonyms: | meterelin;1-9-Luteinizing hormone-releasing factor (pig) , 6-(2-methyl-D-tryptophan)-9-(N-ethyl-L-prolinamide)-;2-Me-D-Trp(6),desgly(10)-lhrh ethylamide;Lhrh ethylamide, 2-me-D-Trp(6),desgly(10)-;1-9-Luteinizing hormone-releasing factor (swine), 6-(2-methyl-D-tryptophan)-9-(N-ethyl-L-prolinamide)-;EP 23904;EP23904;EP-23904 | | CAS: | 140703-49-7 | | MF: | C65H85N17O12 | | MW: | 1296.48 | | EINECS: | | | Product Categories: | | | Mol File: | 140703-49-7.mol | 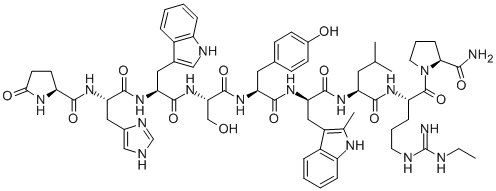 |
| | meterelin Chemical Properties |
| density | 1.47±0.1 g/cm3(Predicted) | | pka | 9.82±0.15(Predicted) |
| | meterelin Usage And Synthesis |
| Originator | Avolerin,Mediolanum | | Manufacturing Process | It is well known that the incorporation or substitution of a D-tryptophan
residue into a biologically active peptide chain enhances the activity of that
chain. Furthermore, such incorporation or substitution will prolong the
biological activity. The prolonged and enhanced effectiveness of such peptides
probably relates the increased resistance to degradation by peptidases.
[D-2-Methyl-Trp6] LHRH and [Des-Gly10-D-2-methyl-Trp6-Pro-ethylamide9] LHRH.
2-Methyl-tryptophan is known (cf. H.N. Rydon, J. Chem. Soc. 1948, 705) and
the homologous alkylated derivatives are conveniently prepared from the
corresponding 2-alkyl indoles by well known methods (cf. J.P. Li et al.,
Synthesis (1), 73, 1988). The resolution of the racemic tryptophan derivatives
to give the D-enantiomers can also be achieved by a variety of methods (cf.
Amino Acids, Peptides and Proteins, Vol. 16, pages 18-20, The Royal Society
of Chemistry, London, 1985). The both solution phase or the solid phase
method of peptide synthesis can be used to make 5-oxo-L-prolyl-L-histidyl-Ltryptophyl-
L-seryl-L-tyrosyl-2-methyl-D-tryptophyl-L-leucyl-L-arginyl-N-ethyl-
L-prolinamide (cf. R. Geiger et al., "The Peptides", Academic Press, New York
1981). If the solid phase method is used, peptide synthesizers such as the
Applied Biosystem 430A, Bioresearch Sam 9500 or the Beckman Model 990
are preferably used. According to this methodology, the first amino acid is
linked to the benzhydrylamine resin and the remaining protected amino acids
are then coupled in a stepwise manner using the standard procedures
recommended by the manufacturers of the synthesizers.
For instance, amino acid couplings are performed by using symmetrical
anhydrides in the Applied Biosystems Synthesizer and diisopropylcarbodiimide
in the Bioresearch or Beckman machines. The amino acid derivatives are
protected by the tertiary butoxy-carbonyl groups on the α-amino function
during the synthesis. The functional groups present in the amino-acid in the
side chain are previously protected. For instance, the functional groups of
histidine are protected by benzyloxymethyl (His(Bom)), tosyl (His(Tos)), the
functional groups of tryptophan by formyl (Trp(For)), those of serine by benzyl
(Ser(Bzl)), those of tyrosine by 2-Br-benzyloxycarbonyl (Tyr(2-Br-Z)), those of
arginine by tosyl (Arg(Tos)), those of proline by O-benzyl HCl (Pro(OBzl HCl)).
The Boc protective groups on the α-aminic function are removed at each stage
by treatment with 60% trifluoroacetic acid ("TFA") in dichloromethane. The
crude peptides after HF cleavage are purified on a Sephadex G-50 F column in
50% acetic acid or by preparative reverse phase HPLC using gradients of
acetonitrile and water containing 0.1% trifluoroacetic acid. | | Therapeutic Function | LHRH agonist |
| | meterelin Preparation Products And Raw materials |
|