|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 2-ヒドロキシベンゾアート | | 英語化学名: | Sodium salicylate | | 别名: | Sdium salicylate;Sodium Salicylate 2-Hydroxybenzoic acid monosodium salt Sodium o-hydroxybenzoate;Sodium salicylate BP98;SODIUM SALICYLATE R. G., REAG. PH. EUR.;SODIUM SALICYLATE, REAGENTPLUS TM, >= 99.5%;SODIUM SALICYLATE, PH EUR;SODIUM SALICYLATE, REAGENTPLUS, >=99%;SodiumSalicylatePurified | | CAS番号: | 54-21-7 | | 分子式: | C7H5NaO3 | | 分子量: | 160.1 | | EINECS: | 200-198-0 | | カテゴリ情報: | API;IDOCYL;Pharmaceutical intermediate;54-21-7 | | Mol File: | 54-21-7.mol | 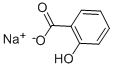 |
| 融点 | >300 °C (lit.) | | 比重(密度) | 0.32 g/cm3 (20℃) | | 貯蔵温度 | Store at +15°C to +25°C. | | 溶解性 | 1000g/l | | 外見 | Crystals or Crystalline Powder | | 色 | White | | 臭い (Odor) | wh. lustrous cryst. scales or amorphous powd., odorless or faint odor, saline taste | | PH | 6.5 (100g/l, H2O, 20℃) | | 水溶解度 | 1000 g/L (20 ºC) | | Sensitive | Light Sensitive | | Merck | 14,8332 | | BRN | 3732792 | | 安定性: | Stable. Incompatible with mineral acids, metallic salts, iodine. May be light-sensitive. | | InChIKey | ABBQHOQBGMUPJH-UHFFFAOYSA-M | | LogP | 2.061 (est) | | CAS データベース | 54-21-7(CAS DataBase Reference) | | EPAの化学物質情報 | Sodium salicylate (54-21-7) |
| 主な危険性 | Xn,Xi | | Rフレーズ | 22-41-36/37/38-36 | | Sフレーズ | 26-36 | | WGK Germany | 1 | | RTECS 番号 | VO5075000 | | F | 8 | | 自然発火温度 | >250 °C | | TSCA | Yes | | HSコード | 29182100 | | 毒性 | LD50 i.p. in rats: 780 mg/kg (Goldenthal) |
| | 2-ヒドロキシベンゾアート Usage And Synthesis |
| 外観 | 白色, 結晶~結晶性粉末 | | 定義 | 本品は、サリチル酸(*)のナトリウム塩であり、次の化学式で表される。
参照表示名称:サリチル酸 | | 溶解性 | 水に易溶 (115.4g/100g, 25℃)。メタノール, エタノールに可溶。水に極めて溶けやすく、エタノールに溶けやすい。水に極めて溶けやすく、エタノールに溶けやすい。 | | 解説 | サリチル酸ナトリウム,光により淡赤色となり,メタノール,エタノール,水に可溶.水溶液はやや酸性を示す.この正塩をサリチル酸水溶液から再結晶すると酸性塩NaC7H5O3・C7H6O3が得られる.また,正塩をそのまま減圧蒸留するとジナトリウム塩Na2C7H4O3が得られる.解熱・鎮痛剤のほか,のり,にかわなどの保存用薬品として用いられる.LD50 1.6 g/kg(マウス,経口).
森北出版「化学辞典(第2版)
| | 用途 | 糊、ゴム糊等の防腐、保存剤 | | 用途 | 鉄(III)、ウランの検出試薬。 | | 用途 | サリチル酸のナトリウム化合 物です。プロスタグランジン生合成阻害作用 を示します。 | | 用途 | シクロオキシゲナーゼ1
(COX-1)を選択的に阻害し、プロスタグラ
ンジン生合成阻害作用を示します。 | | 用途 | シクロオキシゲナーゼ1
(COX-1)を選択的に阻害します。 | | 化粧品の成分用途 | 変性剤、防腐剤 | | 製造 | やや過剰のサリチル酸と炭酸ナトリウムとを水溶液中で加熱すると,分解点260~261 ℃ の鱗(りん)状結晶の正塩が得られるサリチル酸ナトリウム. | | 効能 | 鎮痛薬, 抗炎症薬, シクロオキシゲナーゼ阻害薬 | | 説明 | Sodium salicylate is a sodium salt of salicylic acid. It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure. Historically, it has been synthesized from methyl salicylate (found in wintergreen plants or the bark of sweet birch tree) by reacting it with an excess of sodium hydroxide and heating it under reflux. | | 化学的特性 | Sodium salicylate is of the salicylate family and this compound is known to trigger Reye's Syndrome in children and adults, usually following a viral infection such as influenza or chicken pox. Products containing such salicylates should not be given to children under the age of 19. | | 化学的特性 | Sodium salicylate, HOC6H4COONa, is a shiny, white powder with sweetish taste and mild aromatic aroma that is soluble in water,glycerol, and alcohol.Used in medicine and as a preservative. | | 使用 | In manufacture of aspirin, methyl salicylate, and other salicylates. Coupling agent for azo dyes. Has been used as food preservative. | | 使用 | It is used in medicine as an analgesic and antipyretic. Sodium salicylate also acts as non - steroidal anti - inflammatory drug (NSAID), and induces apoptosis in cancer cells and also necrosis. It is also a potential replacement for aspirin for people sensitive to it. It may also be used as a phosphor for the detection of vacuum ultra violet radiation and electrons. | | 使用 | keratolytic | | 定義 | A salt or
ester of salicylic acid. | | brand name | Alysine (Marion Merrell Dow). | | 一般的な説明 | Sodium salicylate may be prepared by the reaction, in aqueoussolution, by adding equal molar ratio of salicylic acidand sodium bicarbonate; evaporating to dryness yields thewhite salt. In solution, particularly in the presence of sodiumbicarbonate, the salt will darken on standing. This is the saltof choice for salicylate medication and usually is administeredwith sodium bicarbonate to lessen gastric distress, or itis administered in enteric coated tablets. However, the useof sodium bicarbonate is ill advised, because it decreases theplasma levels of salicylate and increases the excretion offree salicylate in the urine.
Sodium salicylate, even though not as potent as aspirinfor pain relief, also has less GI irritation and is useful for patientswho are hypersensitive to aspirin. | | Biochem/physiol Actions | Cell permeable: yes | | 安全性プロファイル | Experimental poison by subcutaneous route. Moderately toxic to humans by ingestion. Moderately toxic experimentally by ingestion, intraperitoneal, and intravenous routes. An experimental teratogen. Human systemic effects by multiple and unspecified routes: toxic psychosis, excitement, respiratory stimulation, nausea or vomiting, and sweating. Experimental reproductive effects. Mutation data reported. A powerful irritant which affects the central nervous system. Incompatible with ferric salts, mineral acids, iodme, lead acetate, silver nitrate, sodium phosphate powder. When heated to decomposition it emits toxic fumes of Na2O. |
|