|
ChemicalBook Optimization Suppliers |
|
| 化学名: | エチニルエストラジオール | | 英語化学名: | Ethinyl Estradiol | | 别名: | 19-NORPREGNA-1,3,5(10)-TRIEN-20-YNE-3,17ALPHA-DIOL;19-NOR-1,3,5[10],17ALPHA-PREGNATRIEN-20-YNE-3,17-DIOL;1,3,5(10)-ESTRATRIEN-17-ALPHA-ETHYNYL-3,17-BETA-DIOL;ACETENYL ESTRADIOL;17ALPHA-ETHYNYL-DELTA1,3,5(10)-ESTRA-TRIENE-3,17BETA-DIOL;17-ALPHA-ETHYNLESTRADIOL;17ALPHA-ETHYNYL-1,3,5[10]-ESTRATRIENE-3,17BETA-DIOL;17a-ethynyl-1,3,5(10)-estratriene-3,17b-diol | | CAS番号: | 57-63-6 | | 分子式: | C20H24O2 | | 分子量: | 296.41 | | EINECS: | 200-342-2 | | カテゴリ情報: | Steroids;Intermediates & Fine Chemicals;Metabolites & Impurities;Pharmaceuticals;Alcohols and Derivatives;Acetylenes;Biochemistry;Functionalized Acetylenes;Hydroxysteroids;Inhibitors;Hormone Drugs;progestogen estrogen;ESTINYL;57-63-6 | | Mol File: | 57-63-6.mol | 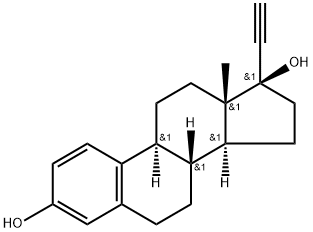 |
| | エチニルエストラジオール Usage And Synthesis |
| 外観 | 白色~うすい黄色~うすい黄赤色粉末~結晶 | | 定義 | 本品は、卵胞ホルモン誘導体であり、次の化学式で表される。 | | 化粧品の成分用途 | 皮膚コンディショニング剤 | | 効能 | 抗悪性腫瘍薬, 月経障害治療薬, エストロゲン受容体作動薬 | | 商品名 | プロセキソール (あすか製薬) | | 説明 | Estrogens direct the development of the female genotype in embryogenesis and at puberty. Estradiol is the major estrogen secreted by the premenopausal ovary. Ethynyl estradiol is a synthetic analog of 17β-estradiol . A USP-approved grade of ethynyl estradiol is often formulated in combination with a progestin such as norgestrel /levonorgestrel or desogestrel and provided for use as an oral contraceptive. Efficacy of oral administration of ethynyl estradiol is facilitated by the ethynyl substitution at the C-17 position, which inhibits first pass hepatic metabolism. Ethynyl estradiol is also rapidly and almost completely absorbed from the gastrointestinal tract. | | 化学的特性 | Off-White to Light-Yellow Crystalline Powder | | 化学的特性 | Estradiol, 17-β-is an odorless white to yellow
crystalline substance. | | 化学的特性 | Ethinylestradiol is a white to creamy-white
powder. Odorless. | | Originator | Estinyl,Schering,US,1944 | | 使用 | A synthetic steroid with high oral estrogenic potency | | 使用 | estrogen, plus progestogen as oral contraceptive | | 使用 | A synthetic estradiol analog. | | 使用 | A metabolite of 17a-Ethynylestradiol | | 定義 | ChEBI: A 3-hydroxy steroid that is estradiol substituted by a ethynyl group at position 17. It is a xenoestrogen synthesized from estradiol and has been shown to exhibit high estrogenic potency on oral administration. | | Manufacturing Process | In about 250 cc of liquid ammonia (cooled with dry ice and acetone) are
dissolved about 7.5 g of potassium and into the solution acetylene is passed
until the blue color has disappeared (about 3 hours). Then slowly a solution or
suspension of 3 g of estrone in 150 cc of benzene and 50 cc of ether is added.
The freezing mixture is removed, the whole allowed to stand for about 2
hours and the solution further stirred overnight. Thereupon the reaction
solution is treated with ice and water, acidified with sulfuric acid to an acid
reaction to Congo red and the solution extracted five times with ether. The
combined ether extracts are washed twice with water, once with 5% sodium
carbonate solution and again with water until the washing water is neutral.
Then the ether is evaporated, the residue dissolved in a little methanol and
diluted with water. The separated product is recrystallized from aqueous
methanol. The yield amounts to 2.77 g. The 17-ethinyl-estradiol-3,17 thus obtained melts at 142°C to 144°C. | | brand name | Estinyl (Schering); Feminone
(Pharmacia & Upjohn); Lynoral (Organon). | | Therapeutic Function | Estrogen | | 一般的な説明 | 17 -Ethinyl estradiol has thegreatest advantage over other estradiol products of beingorally active. It is equal to estradiol in potency by injectionbut is 15 to 20 times more orally active. The primary metabolicpath for ethinyl estradiol is 2-hydroxylation bycytochrome P450 isozyme 3A4 (CYP3A4), followed byconversion to the 2- and 3-methyl ethers by catechol-Omethyltransferase.The 3-methyl ether of ethinyl estradiolis mestranol, USP, used in oral contraceptives. Mestranolis a prodrug that is 3-O-demethylated to the active ethinylestradiol. An oral dose of about 50 μg of mestranol has anestrogenic action approximately equivalent to 35 g oforal ethinyl estradiol. The demethylation is mainly mediatedby CYP2C9. | | 一般的な説明 | Fine white to creamy white powder. A synthetic steroid. Used in combination with progestogen as an oral contraceptive. | | 空気と水の反応 | Air and light sensitive . Insoluble in water. | | 反応プロフィール | Ethynyl estradiol may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to generate gaseous hydrogen. | | 健康ハザード | ACUTE/CHRONIC HAZARDS: When heated to decomposition Ethynyl estradiol emits acrid smoke and fumes. | | 火災危険 | The flash point data for Ethynyl estradiol are not available. Ethynyl estradiol is probably combustible. | | Biochem/physiol Actions | 17α-Ethynylestradiol is an orally bio-active synthetic estrogen used as an oral contraceptive. | | 作用機序 | Synthetic estrogen with potent
activity (inhibition of ovulation), widely used in oral contraceptives.
Manufactured from natural estrogen, estrone, by reaction
with potassium acetylide (HCRCK) in liquid ammonia.
The synthetic 17α-ethynyl derivative of estradiol-17β. The
17α-ethynyl group increases the in vivo potency of estradiol-
17β by blocking the action of 17β-dehydrogenase, a major
pathway of estradiol-17β metabolic inactivation. It is thus
active orally and is among the most potent of the known estrogenic
compounds. | | 安全性プロファイル | Confirmed carcinogen
with experimental carcinogenic,
tumorigenic, and neoplastigenic data. Poison
by intraperitoneal route. Moderately toxic by
ingestion. Human systemic effects by
ingestion: glandular effects. An experimental
teratogen. Experimental reproductive
effects. Human mutation data reported.
When heated to decomposition it emits
acrid smoke and irritating fumes. See also
ESTRADIOL | | 合成 | Ethinyl estradiol, 17|á-ethinyl-1,3,5(10)-estratrien-3-17|?-diol (28.1.26),
is made either by condensing estrone with acetylene in the presence of potassium hydroxide
(Favorskii reaction), or by reacting sodium acetylenide in liquid ammonia with estrone. 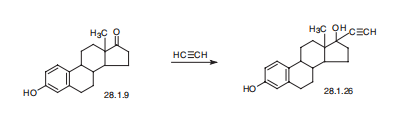
| | 職業ばく露 | The working environment may be
contaminated during sex hormone manufacture, especially
during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered
products and recrystallization. Airborne particles of sex
hormones may be absorbed through the skin, ingested or
inhaled. Enteric absorption results in quick inactivation of
sex hormones in the liver. The rate of inactivation is
decreased for the oral, alkylated steroid hormones (methyl
testosterone, anabolic steroids, etc.). Sex hormones may
accumulate and reach relatively high levels even if their
absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health.
Intoxication by sex hormones may occur in almost all the
exposed workers if preventive measures are not taken. The
effect in the industrial sector is more successful than
the agricultural one (chemical caponizing of cockerels by
stilbestrol implants and incorporation of estrogens in feed
for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger | | 応急処置 | Skin Contact: Flood all areas of body thathave contacted the substance with water. Do not wait toremove contaminated clothing; do it under the water stream.Use soap to help assure removal. Isolate contaminatedclothing when removed to prevent contact by others. EyeContact: Remove any contact lenses at once. Immediatelyflush eyes well with copious quantities of water or normalsaline for at least 20-30 min. Seek medical attention.Inhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing, or any othersymptoms develop, seek medical attention at once, even ifsymptoms develop many hours after exposure. Ingestion:Contact a physician, hospital, or poison center at once. Ifthe victim is unconscious or convulsing, do not inducevomiting or give anything by mouth. Assure that thepatient’s airway is open and lay him on his side with hishead lower than his body and transport immediately to a medical facility. If conscious and not convulsing, give aglass of water to dilute the substance. Vomiting should notbe induced without a physician’s advice. | | 貯蔵 | Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in a refrigerator under an inert atmosphereand protect from exposure to light. | | 輸送方法 | UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials | | 純化方法 | 17--Ethynylestradiol forms a hemihydrate on recrystallising from MeOH/H2O. It dehydrates on melting and remelts on further heating at m 182-184o. The UV has max at 281nm ( 2040) in EtOH. Its solubility is 17% in EtOH, 25% in Et2O, 20% in Me2CO, 25% in dioxane and 5% in CHCl3. [Petit & Muller Bull Soc Chim Fr 121 1951.] The diacetyl derivative has m 143-144o (from MeOH) and [] D 20 +1o (c 1, CHCl3) [Mills et al. J Am Chem Soc 80 6118 1958]. [Beilstein 6 IV 6877.] | | 不和合性 | May react exothermically with reducing
agents to generate flammable gaseous hydrogen.
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases, strong
acids, oxoacids, and epoxides. | | 廃棄物の処理 | It is inappropriate and possibly dangerous to the environment to dispose of expired or
waste drugs and pharmaceuticals by flushing them down
the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed
with wet cat litter or coffee grounds, double-bagged in
plastic, discard in trash. Larger quantities shall carefully
take into consideration applicable DEA, EPA, and FDA
regulations. If possible return the pharmaceutical to the
manufacturer for proper disposal being careful to properly
label and securely package the material. Alternatively, the
waste pharmaceutical shall be labeled, securely packaged
and transported by a state licensed medical waste contractor
to dispose by burial in a licensed hazardous or toxic waste
landfill or incinerator |
|