|
ChemicalBook Optimization Suppliers |
|
| 融点 | >300 °C (lit.) | | 沸点 | 333.35°C (rough estimate) | | 比重(密度) | 1.2319 (rough estimate) | | 屈折率 | 1.6000 (estimate) | | 貯蔵温度 | 2-8°C | | 溶解性 | DMSO: 27 mg/mL | | 外見 | powder | | 酸解離定数(Pka) | 6.53±0.40(Predicted) | | 色 | yellow | | Merck | 14,730 | | BRN | 262620 | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | | InChI | InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | | InChIKey | KZNIFHPLKGYRTM-UHFFFAOYSA-N | | SMILES | C1(C2=CC=C(O)C=C2)OC2=CC(O)=CC(O)=C2C(=O)C=1 | | LogP | 3.020 | | CAS データベース | 520-36-5(CAS DataBase Reference) |
| | アピゲニン Usage And Synthesis |
| 外観 | 白色〜褐色, 結晶〜粉末 | | 定義 | 本品は、次の化学式で表される複素環式化合物である。 | | 溶解性 | ジメチルスルホキシド、N,N-ジメチルホルムアミド及びアセトンに溶け、水にほとんど溶けない。 | | 解説 | 4′,5,7-trihydroxyflavone.C15H10O5(270.24).フラボンの誘導体で,ダリア,フジモドキなどの花に含まれる黄色の色素.配糖体をアピインといい,これはパセリ,セロリの葉などに含まれる. フラボン色素の一種.植物体では,遊離状または,配糖体として存在し,パセリApium petroselinum L.のアピイン(C26H28O14,7-アピオグルコシド),コスモスの白花に含まれるコスモシイン(C21H20O10,7-グルコシド),ハゼノキの葉やカラタチの葉,花にあるロイフォリン(C27H30O14,7-ラムノグルコシド)などがある.アピゲニンは,淡黄色の小片状の結晶.融点347 ℃.エタノールに易溶,エーテル,熱水に難溶.アルカリ溶液は鮮黄色,濃硫酸には黄色に溶けて弱い緑色の蛍光を発する.塩化鉄(Ⅲ)で黒褐色を呈する.森北出版「化学辞典(第2版) | | 用途 | 情報伝達研究用。 | | 化粧品の成分用途 | ヘアコンディショニング剤、酸化防止剤 | | 化学的特性 | Pale Yellow Crystalline Solid | | 使用 | Apigenin has been shown to possess antibacterial, antiviral, antifungal, and antiparasitic activities. Although it can’t stop all types of bacteria on its own, it can be combined with other antibiotics to increase their effects. | | 使用 | Apigenin is a promising reagent for cancer therapy. Apigenin appears to have the potential to be developed either as a dietary supplement or as an adjuvant chemotherapeutic agent for cancer therapy. | | 使用 | Apigenin is an active antioxidant, anti-inflammatory, anti-amyloidogenic, neuroprotective and cognitive enhancing substance with interesting potential in the treatment/prevention of Alzheimer's disease. | | 製造方法 | 4-hydroxybenzaldehyde (1.22 g, 9.97 mmol, 1.0 equiv) was added to asolution of 50% KOH (aq.) (6.72 g, 59.82 mmol, 6.0 equiv) and ethanol (3 mL) andstirred for 10 min. Then compound 9 (2.02g, 9.97 mmol, 1.0 equiv) was added to the reaction mixture and heated to 60 °C and stirred for 4 h. After cooled toroom temperature, the mixture was poured into ice water and acidified withconcentrated hydrochloric acid to pH = 3. Then the suspension was filtrated, washed and the residue was dried toafford Apigenin(2.43 g, 90%) as a red solid. 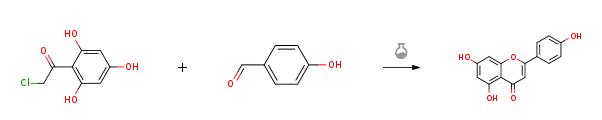
| | 定義 | ChEBI: Apigenin is a trihydroxyflavone that is flavone substituted by hydroxy groups at positions 4', 5 and 7. It induces autophagy in leukaemia cells. It has a role as a metabolite and an antineoplastic agent. It is a conjugate acid of an apigenin-7-olate. | | 利点 | Apigenin has various beneficial health effects such as antioxidant, anti-inflammatory, and chemoprevention. Apigenin can downregulate the expression of IL-1β and TNF-α in LPS-stimulated mouse macrophages and human monocytes.
preclinical studies have suggested that apigenin may improve outcomes in multiple health states, including anxiety, brain function, oxidative stress, inflammation, and hormonal regulation (testosterone, estrogen, and cortisol | | 作用機序 | Apigenin exerts anxiolytic effects at high doses by inhibiting NMDA receptors; it also has affinity to GABA-A receptors. Apigenin also exerts potent antioxidant activities by scavenging free radicals and upregulating glutathione levels; it also exerts anti-inflammatory effects. Apigenin is most studied for its potential anti-cancer properties. | | 抗がん研究 | It induces apoptosis by targeting leptin/leptin receptor pathway and by targeting caspase-dependent extrinsic pathway aswell as STAT3 signaling pathway in lung adenocarcinoma and BT-474 breast cancercells, respectively.
It shows antitumor activity against breastcancer MCF-7 cells and colon cancer HCT 116 cells and is a mediator of cancerchemoprevention and an inducer of autophagy. It can be used to treat colon canceras it induces apoptosis in colon cancer cells. It also increase melanogenesis in B16cells by activating the p38 MAPK pathway. | | 安全性 | Apigenin is considered safe when consumed in normal amounts through a diet rich in fruits, vegetables, and herbs. However, supplement doses tend to deliver a significantly higher amount of apigenin than would be generally consumed via dietary means. Higher doses of apigenin can cause stomach discomfort, and people should cease using it immediately and consult medical professionals. At high doses, it can trigger muscle relaxation and sedation[1]. Allergic reactions can also occur as a response to chamomile tea or apigenin.
| | Source | Apigenin (4′,5,7-trihydroxyflavone) is one of the most widespread flavonoids in plants and formally belongs to the flavone sub-class. Plants belonging to the Asteraceae, such as those belonging to Artemisia, Achillea, Matricaria, and Tanacetum genera, are the main sources of this compound. However, species belonging to other families, such as the Lamiaceae, for instance, Sideritis and Teucrium, or species from the Fabaceae, such as Genista, showed the presence of apigenin in the aglycone form and/or its C- and O-glucosides, glucuronides, O-methyl ethers, and acetylated derivatives. In gymnosperms, apigenin derivatives are mostly present in dimeric forms, with apigenin residues variously coupled, e.g., with C?C linkage as in cupressuflavone and amentoflavone (I-8, II-8″ and I-3′, II-8″, respectively), or C—O linkage (I-4′, II-6″) as in hinokiflavone. | | 貯蔵 | +4°C | | 純化方法 | The current method for the purification of apigenin is crude-solvent extraction method by using solvent in small quantity and also time saving. Apigenin that was isolated from Symphyotrichum novae-angliae was obtained in large quantity and directly from extract. | | 参考文献 | [1] Bahare Salehi, et al. “The Therapeutic Potential of Apigenin.” Int J Mol Sci. 2019 Mar; 20(6): 1305.
| | 参考文献 | [1] Sanjeev Shukla, Sanjay Gupta (2010) Apigenin: a promising molecule for cancer prevention, 27, 962-978.
[2] https://en.wikipedia.org/wiki/Apigenin
[3] http://bodynutrition.org/apigenin/ |
|