|
ChemicalBook Optimization Suppliers |
| 名前: |
Sigma-Aldrich |
| 電話番号: |
021-61415566 800-8193336 |
| 電子メール: |
orderCN@merckgroup.com |
|
| | (S)-3,4,5,6,9,10,11,12-オクタヒドロ-14,16-ジヒドロキシ-3-メチル-1H-2-ベンゾオキサシクロテトラデシン-1,7(8H)-ジオン 製品概要 |
| 化学名: | (S)-3,4,5,6,9,10,11,12-オクタヒドロ-14,16-ジヒドロキシ-3-メチル-1H-2-ベンゾオキサシクロテトラデシン-1,7(8H)-ジオン | | 英語化学名: | ZEARALANONE | | 别名: | (S)-Zearalanone, P 1502, Zanone;(S)-3,4,5,6,9,10,11,12-Octahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione;(S)-Zearalanone;2-[(S)-10-Hydroxy-6-oxoundecyl]-4,6-dihydroxybenzoic acid 1,2-lactone;P-1502;Zanone;1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10,11,12-octahydro-14,16-dihydroxy-3-methyl-, (S)-;beta-Resorcylic acid, 6-(10-hydroxy-6-oxoundecyl)-, mu-lactone | | CAS番号: | 5975-78-0 | | 分子式: | C18H24O5 | | 分子量: | 320.38 | | EINECS: | | | カテゴリ情報: | Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Metabolites & Impurities;Pharmaceuticals | | Mol File: | 5975-78-0.mol | 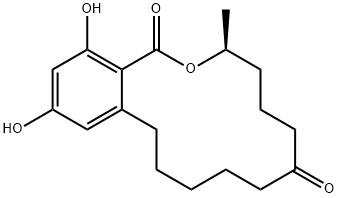 |
| | (S)-3,4,5,6,9,10,11,12-オクタヒドロ-14,16-ジヒドロキシ-3-メチル-1H-2-ベンゾオキサシクロテトラデシン-1,7(8H)-ジオン 物理性質 |
| 融点 | 184-186°C | | 沸点 | 576.8±50.0 °C(Predicted) | | 比重(密度) | 1.148±0.06 g/cm3(Predicted) | | 闪点 | 2℃ | | 貯蔵温度 | -20°C | | 溶解性 | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | 外見 | Solid | | 酸解離定数(Pka) | 7.83±0.40(Predicted) | | 色 | White to Off-White |
| | (S)-3,4,5,6,9,10,11,12-オクタヒドロ-14,16-ジヒドロキシ-3-メチル-1H-2-ベンゾオキサシクロテトラデシン-1,7(8H)-ジオン Usage And Synthesis |
| 化学的特性 | White to Off-White Solid | | 使用 | A secondary metabolite of Zearalenone (ZON). The macrocyclic lactone Zearalanone used in the synthesis of d,l-Zearalanol. | | 使用 | Zearalanone is a minor component of the zearalenone complex produced by several species of Fusarium. Like the more abundant analogues, zearalanone causes estrogenic effects in domestic livestock. Zearalanone is a metabolite of α-zearalanol, a commercially available growth promotant in animals, and is a useful standard for detection of zearanol-contaminated products and Fusarium-contaminated grains. | | 定義 | ChEBI: Zearalanone is a member of resorcinols and a macrolide. | | 一般的な説明 | Zearalanone (ZEA) or F-2 toxin, a nonsteroidal estrogenic mycotoxin is produced by various Fusarium species. Fusarium graminearum is considered as the primary producer of zearalanone. It is usually seen in moldy hay, high-moisture corn, corn infected before harvest etc. | | 生物活性 | zearalanone (zan) is an estrogen receptor agonist and also an androgen receptor antagonist [1].zearalenone (zen), a β-resorcyclic acid lactone (ral), is a non-steroidal estrogenic mycotoxin biosynthesized by several fusarium species. zearalenone (zen) is the well-known mycotoxin present in numerous agricultural products [1][2][3][4]. in the human endometrial ishikawa cell line, zearalenone (zen) exhibited strong oestrogenicity [4].when incubated fusarium semitectum on sorghum, the ratio of zan to zen remained constant after 5 days, suggesting that zan might not be suitable for use as an internal standard [5].zearalanone, a major phase i metabolite of zearalenone (zen), is an estrogen receptor agonist and also an androgen receptor antagonist. in mcf-7 cells, zearalanone strongly induced cell proliferation with ec50 value of 1.21 nm and showed significant estrogenic activity. zearalanone behaved as full herα agonist. in palm cells, zearalanone acted as a har antagonist that strongly inhibited the luciferase activity induced by 0.3 nm of r1881, the synthetic androgen [1]. | | Biochem/physiol Actions | Zearalanone is believed to cause vulvovaginitis and estrogenic responses in swine. | | 参考文献 | [1]. molina-molina jm, real m, jimenez-diaz i, et al. assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. food chem toxicol.2014 dec;74:233-9.
[2]. baldwin rs, williams rd, terry mk. zeranol: a review of the metabolism, toxicology, and analytical methods for detection of tissue residues. regul toxicol pharmacol. 1983 mar;3(1):9-25.
[3]. migdalof bh, dugger ha, heider jg, et al. biotransformation of zeranol: disposition and metabolism in the female rat, rabbit, dog, monkey and man. xenobiotica. 1983 apr;13(4):209-21.
[4]. le guevel r, pakdel f. assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. hum reprod. 2001 may;16(5):1030-6.
[5]. aoyama k, ishikuro e, noriduki h, et al. formation ratios of zearalanone, zearalenols, and zearalanols versus zearalenone during incubation of fusarium semitectum on sorghum and ratios in naturally contaminated sorghum. shokuhin eiseigaku zasshi. 2015;56(6):247-51. |
|