| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:Cephapirin
CAS:21593-23-7
Package:250Mg,25Mg
|
|
| | cefapirin Basic information |
| Product Name: | cefapirin | | Synonyms: | 5-Thia-1-azabicyclo4.2.0oct-2-ene-2-carboxylic acid, 3-(acetyloxy)methyl-8-oxo-7-(4-pyridinylthio)acetylamino-, (6R,7R)-;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-(hydroxymethyl)-8-oxo-7-[2-(4-pyridylthio)acetamido]-, acetate (ester) (8CI);5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(acetyloxy)methyl]-8-oxo-7-[[(4-pyridinylthio)acetyl]amino]-, (6R-trans)-;7-[2-(4-Pyridylthio)acetamido]cephalosporanic acid;Cefaprin;C06896;(6R,7R)-3-[(Acetyloxy)Methyl]-8-oxo-7-[[(4-pyridinylthio)acetyl]aMino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid;(6R-trans)-3-[(Acetyloxy)Methyl]-8-oxo-7-
[[(4-pyridinylthio)acetyl]aMino]- | | CAS: | 21593-23-7 | | MF: | C17H17N3O6S2 | | MW: | 423.46 | | EINECS: | 244-466-5 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Aromatics;Chiral Reagents;Heterocycles | | Mol File: | 21593-23-7.mol | 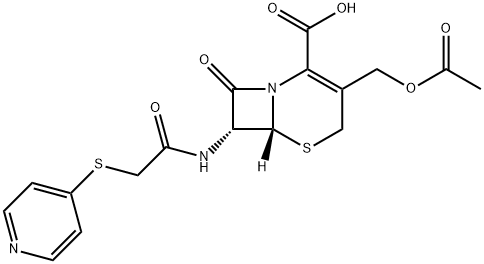 |
| | cefapirin Chemical Properties |
| Melting point | >135°C (dec.) | | Boiling point | 783.9±60.0 °C(Predicted) | | density | 1.4021 (rough estimate) | | refractive index | 1.6390 (estimate) | | storage temp. | -20°C Freezer, Under Inert Atmosphere | | solubility | ≤10mg/ml in DMSO;5mg/ml in dimethyl formamide | | form | crystalline solid | | pka | 2.67±0.50(Predicted) | | CAS DataBase Reference | 21593-23-7 |
| | cefapirin Usage And Synthesis |
| Chemical Properties | Beige Solid | | Originator | Cefadyl,Bristol,US,1974 | | Uses | An antimicrobial agent. | | Definition | ChEBI: Cephapirin is a cephalosporin with acetoxymethyl and 2(pyridin-4-ylsulfanyl)acetamido substituents at positions 3 and 7, respectively, of the cephem skeleton. It is used (as its sodium salt) as an antibiotic, being effective against gram-negative and gram-positive organisms. It has a role as an antibacterial drug. It is a conjugate acid of a cephapirin(1-). | | Manufacturing Process | One route is that described in US Patent 3,422,100 as follows, starting with
aminocephalosporanic acid (ACA): 27.2 g (0.1 mol) of 7-ACA, 33.2 g (0.3
mol) of NaHCO3, 200 ml of water and 100 ml of acetone were mixed together,
cooled to 0°C and stirred rapidly while 20.1 g (0.1 mol) of bromoacetyl
bromide dissolved in 100 ml of acetone was added in one fast addition. The
temperature was kept at 0 to 5°C for ten minutes, then the ice-salt bath was
removed and stirring continued for one hour as the temperature approached
25°C. The mixture was concentrated in vacuo at 20°C to one-half volume and
200 ml of water added. Two 400 ml ether extracts were made and discarded.
The aqueous solution was covered with 200 ml of ethyl acetate and vigorously
stirred and cooled while being acidified to pH 2 with 40% phosphoric acid.
The mixture was filtered, the ethyl acetate layer separated and washed with
three 100 ml portions of water, dried over Na2SO4, filtered and treated with
30 ml of sodium 2-ethylhexanoate in n-butanol (34 ml = 0.1 mol). The oil
which settled out was scratched to induce crystallization. After stirring for 20 minutes the product, sodium 7-(α-bromoacetamido)cephalosporanate, was
scraped from the sides of the flask and collected. The filter cake was washed
with several portions of acetone, air dried, and dried in vacuo over P2O5.The
yield was 22.5 g and decomposed at 193°C.
A solution of 1.13 g (0.01 mol) of 2-mercaptopyrimidine and 1.06 g (0.01
mol) of sodium carbonate dissolved in 25 ml of water was added dropwise
over a period of an hour at room temperature, to a stirred solution of 4.15 g
(0.01 mol) of sodium 7-(α-bromoacetamido)cephalosporanate in 25 ml of
water.
Stirring was continued an additional 90 minutes and then 50 ml of ethyl
acetate was added, Forty percent H3PO4 was added dropwise with vigorous
stirring until pH 2.5 to 3 was obtained. The product crystallized immediately
and was filtered off, washed several times with water and then three times
with 25 ml portions of ethyl acetate, following which it was air dried. The yield
was 2.9 g of crystals that decomposed at 167 to 168°C. The IR and NMR
spectra were consistent with the desired product, 7-[α-(2-pyrimidinylthio)
acetamido]-cephalosporanic acid monohydrate.
An alternate route is that described in US Patent 3,503,967 which uses ACA in
the last step.
Another alternative route is that described in US Patent 3,578,661 uses
bromomethylcephalosporin as one raw material.
However the acid is prepared, the sodium salt may be prepared as described
in US Patent 3,503,967: Five liters of methylene chloride were added to a
clean dry vessel equipped with stirrer. 7-[α(4-pyridylthio)acetamido]
cephalosporanic acid (1,000 g) was added to the vessel, followed by 350 ml of
triethylamine. The resultant solution was treated with decolorizing charcoal for
15 minutes and filtered. A solution of sodium-3-ethyl-hexanoate (27.3%) in
butanol-methylene chloride was added to the filtrate with stirring. Seven
thousand five hundred milliliters of acetone was added. Crystallization
occurred while stirring was continued several hours under dry conditions. The
crystals were collected by filtration, washed with large volumes of acetone,
and then dried in vacuo at 50°C to yield about 950 g of the title compound. | | Brand name | Cefadyl (Apothecon). | | Therapeutic Function | Antibacterial | | Biological Activity | cefapirin is the first generation cephalosporin antibiotic. cefapirin is broadly effective against gram-negative and gram-positive bacteria [1].the antibacterial action of cefapirin was due to the inhibition of cell-wall synthesis, mediated by its binding to penicillin binding proteins. it was mainly used in veterinary medicine for intramammary treatment of mastitis and intrauterine treatment of endometritis in cattle [1,2].cefapirin was an antibiotic particularly suitable for the treatment of acute otitis media. in 210 strains isolated from the auricular exudate of childrens' acute otitis media, the mic50 and mic90 of cefapirin were 2 and 4 mg/l for 112 strains of haemophilus. the mic ranged from 0.25 to 4 mg/l for ten strains of branhamella catarrhalis (9 secreted a beta-lactamase). cefapirin had an extremely high activity with mic50 and 90 less than 0.06 mg/l against streptococcus pneumoniae. for the strains of staphylococcus aureus sensitive to meticillin, one had a mic less than 0.06 mg/l, and 11 had a mic of 0.25 mg/l. for 14 strains of enterobacteriaceae studied, the mic 50 was 8 mg/l and the mic 90 was 32 mg/l [3]. | | Safety Profile | Moderately toxic by
intraperitoneal route. Human systemic
effects by intravenous route: jaundice.
Experimental reproductive effects. When
heated to decomposition it emits very toxic
fumes of NOx and SOx. | | references | [1] committee for veterinary medicinal products: cefapririn. emea, 1-6 (2001).
[2] ray, p. ,knowlton, k.f.,shang, c., et al. development and validation of a uplc-ms/ms method to monitor cephapirin excretion in dairy cows following intramammary infusion. plos one 9(11), 1-12 (2014).
[3] simonet m, herrmann j l, gehanno p, et al. activity of cefapirin against bacterial strains isolated from acute otitis media in children[j]. pathologie-biologie, 1990, 38(5): 352-354. |
| | cefapirin Preparation Products And Raw materials |
|