|
ChemicalBook Optimization Suppliers |
|
| 融点 | 196-198°C | | 貯蔵温度 | 2-8°C | | 溶解性 | H2O: soluble30mg/mL, clear | | 外見 | powder | | 色 | white to beige | | 光学活性 (optical activity) | [α]/D -26 to -36°, c = 1 in H2O | | 水溶解度 | Soluble in water (75 mM) | | BCS Class | 1 (CLogP), 3
(LogP) | | InChI | InChI=1/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/s3 | | InChIKey | PGZUMBJQJWIWGJ-IFAKAUOZSA-N | | SMILES | [C@@H]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(=O)C)C(=O)OCC.OP(O)(O)=O |&1:0,10,12,r| | | CAS データベース | 204255-11-8(CAS DataBase Reference) |
| 国連危険物分類 | IRRITANT | | HSコード | 2924299500 |
| 外観 | 白色~ごくうすい黄色、結晶性粉末~粉末又は塊 | | 溶解性 | 水に可溶 | | 医薬用 | タミフルは,A型およびB型インフルエンザ感染症に対する経口抗インフルエンザウイルス薬の製品名。一般名はリン酸オセルタミビル。[幸保文治][参照項目] | リン酸オセルタミビル
小学館 日本大百科全書(ニッポニカ) ) | | 用途 | ノイラミニダーゼ阻害剤です。
代謝により活性体に変換され、インフルエン
ザウイルスのノイラミニダーゼを阻害し、新
たに形成されたウイルスの細胞外への遊離を
阻害することにより、ウイルスの増殖を抑制
します。
| | 効能 | 抗ウイルス薬, ノイラミニダーゼ阻害薬 | | 商品名 | タミフル (中外製薬); タミフル (中外製薬) | | 説明 | Oseltamivir phosphate (Tamiflu) was launched in the US and Switzerland for the treatment of influenza infections by all common strain viruses. It is an oral anti-viral drug approved for the treatment of acute, uncomplicated influenza in patients 2 weeks of age and older whose flu symptoms have not lasted more than two days. This product is approved to treat Type A and B influenza; however, the majority of patients included in the studies were infected with type A, the most common in the U.S. Efficacy of Tamiflu in the treatment of influenza in subjects with chronic cardiac disease and/or respiratory disease has not been established. | | 化学的特性 | White Cyrstalline Solid. It is freely soluble in water. | | Originator | Gilead (US) | | 使用 | Oseltamivir phosphate (Tamiflu) is a competitive neuraminidase inhibitor. The prodrug oseltamivir phosphate (Tamiflu) is itself not virally effective; however, once in the liver, it is converted by natural chemical processes, hydrolysed hepatically to its | | 製造方法 | Oseltamivir phosphate (Tamiflu) has been synthesized from cis-2,3-bis(hydroxymethyl)aziridine. After protection of the cis-2,3-bis(hydroxymethyl)aziridine with a Boc group, desymmetrization provided a chiral aziridine, which was a key intermediate to install the required stereogenic center containing a nitrogen atom. Allylation and ring closing metathesis are the key reactions to obtain the cyclic product that was successfully converted to the desired oseltamivir phosphate. DOI: 10.1021/jo3015853
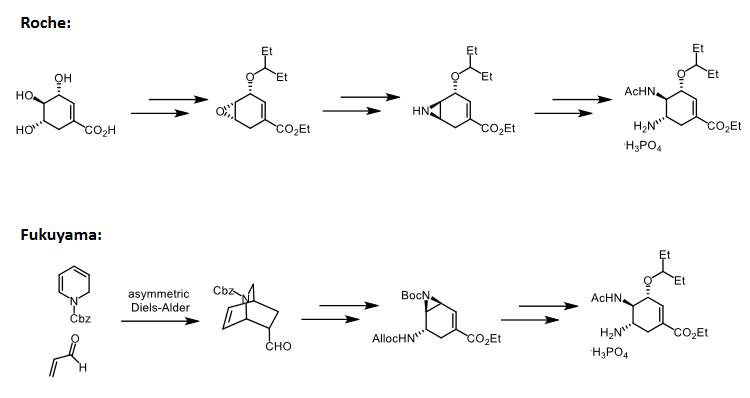
It can also be obtained by a novel 12-step synthesis from (-)-quinic acid. | | 定義 | ChEBI: Oseltamivir phosphate is a phosphate salt. It contains an oseltamivir. It is an acetamido cyclohexene that is a structural homolog of SIALIC ACID and inhibits NEURAMINIDASE. | | brand name | Tamiflu (Roche). | | Therapeutic Function | Antiviral | | 一般的な説明 | receptor site showed clearly that additional binding sitesexist for the C-5 acetamido carbonyl group and the arginineresidue at position 152 of the receptor site. In addition, the C-2 carboxyl group of sialic acid binds to Arg 118, Arg 292, andArg 371. Position C-6 is capable of undergoing a hydrophobicinteraction with various amino acids, including Glu, Ala,Arg, and Ile. Maximum binding to neuraminidase occurswhen the C-6 substituent is substituted with a nonpolar chain.In oseltamivir, this nonpolar group is 3-pentyl. An importantfeature of oseltamivir is the ethyl ester, which makes the drugorally efficacious. This drug is the first orally active agent foruse against influenza A and B. It is also indicated for the treatmentof acute illness. If administered within 2 days after theonset of influenza symptoms, the drug is effective. | | Biochem/physiol Actions | Oseltamivir phosphate is an influenza viral neuraminidase inhititor. Oseltamivir phosphate, an antiviral, is used clinically to treat influenza A and influenza B, and to prevent flu after exposure. Oseltamivir phosphate is hydrolyzed in the liver to its active form, oseltamivir carboxylate, which is an inhibitor of influenza viral neuraminidases essential for viral replication. Oseltamivir has a broad spectrum of activity against a range of influenza A and B subtypes with IC50 values for neuraminidases measured from less than 1 nM to approximately 30 nM, depending on the virus subtype. | | 臨床応用 | Oseltamivir was approved as the first orally administered neuraminidase inhibitor used against influenza A and B viruses. The drug is indicated for the treatment of uncomplicated acute illness caused by influenza infection. | | 副作用 | Side effects with oseltamivir are minor, consist of nausea and vomiting, and occur primarily in the first two days of therapy. | | Veterinary Drugs and Treatments | Although, there is no research published (at the time of writing—
January 2007) documenting oseltamivir safety or efficacy in dogs
or cats, there is much interest and discussion regarding its potential
for the adjunctive treatment of parvovirus infections in dogs.
It may be of benefit for adjunctive treatment of other viral infections,
particularly those with associated secondary bacterial components,
but research or experience is lacking. A recent study performed
in horses, experimentally infected with equine influenza A
(H3N8), documented some efficacy in the attenuation of clinical
signs (pyrexia), viral shedding, and secondary bacterial pneumonias
(Yamanaka, Tsujimura et al. 2006).
Because oseltamivir is the primary antiviral agent proposed for
treatment or prophylaxis for an H5N1 influenza (“bird flu”) pandemic
in humans, its use in veterinary patients is controversial, particularly
due to concerns of adequate drug supply for the human
population and the potential for influenza virus resistance development.
In 2006, the FDA banned the extra-label use of oseltamivir
and other influenza antivirals in chickens, turkeys and ducks. At
the time of writing, its use is still allowed in mammal veterinary
patients, but veterinarians should use the drug prudently and be
cognizant of these public health concerns. | | 代謝 | Oseltamivir is readily absorbed from the GI tract following oral administration. It is a prodrug that is extensively metabolized in the liver, undergoing ester hydrolysis to the active carboxylic acid. Two oxidative metabolites also have been isolated, with the major oxidation product being the ω-carboxylic acid. | | 貯蔵 | -20°C | | Mode of action | Oseltamivir Phosphate is the phosphate salt of oseltamivir, a synthetic derivative prodrug of ethyl ester with antiviral activity. By blocking neuraminidases on the surfaces of influenza viruses, oseltamivir interferes with host cell release of complete viral particles. | | Clinical claims and research | Oseltamivir is the ethyl ester prodrug of GS-4071, the corresponding acid, which is one of the most potent inhibitors of both influenza A and B virus neuraminidase (sialidase) isoenzymes; these glycoproteins are expressed on the virion surface and are essential for virus replication for both A and B strains. Oseltamivir emerged as one of the first two neuraminidase inhibitors to reach the market. GS-4071 demonstrated a low (< 5%) oral bioavailability in animals due to a poor absorption from the gastrointestinal barrier; by incorporating a more lipophilic ester group, the oral bioavailabilty can reach 30 to 100% in mice, rats and dogs. Following oral administration of Oseltamivir in rats, a similar concentration of GS-4071 was found in the bronchoalveolar lining fluid and the plasma which indicated a good penetration of the active compound into the lower respiratory tract. In mice, chickens and ferrets, orally administered Oseltamivir was found to have significant inhibitory effects on A and B influenza infections in protecting against a lethal challenge of virus and lessening virus titer in the lungs or nasal washings. In several clinical trials with patients receiving oral capsules daily, Oseltamivir was shown to be effective in reducing significantly the duration and severity of the clinical symptoms, including fever, cough and general malaise, in both early treatment and prevention. |
|