|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アセトニトリル | | 英語化学名: | Acetonitrile | | 别名: | ACETONITRILE, 99.93+%, HPLC GRADE;ACETONITRILE SOLUTION, ~10% IN WATER, PACKAGE W. 200 ML*;ACETONITRILE CHROMASOLV FOR HPLC,GRADIENT GRADE;ACETONITRILE WITH 0.035% ACETIC ACID, H&;ACETONITRILE ABSOLUTE OVER MOLECULAR SIE VE (H2O <0.01%);ACETONITRILE E CHROMASOLV FOR HPLC, FAR UV;ACETONITRILE, 50 L PURE-PAC II;B-KETOGLUTARIC ACID | | CAS番号: | 75-05-8 | | 分子式: | C2H3N | | 分子量: | 41.05 | | EINECS: | 200-835-2 | | カテゴリ情報: | Biotech;Biotech Solvents;Organics;Composite Drums;Drums Product Line;NOWPak Products;HPLC Gradient Grade Solvents (CHROMASOLV);PVC Coated Bottles;ACS Grade;ACS Grade Solvents;Carbon Steel Flex-Spout Cans;Core Bioreagents;Life Science Reagents for Protein Electrophoresis;Research Essentials;Anhydrous;Anhydrous Solvents;Sure/Seal Bottles;Alternative Energy;Electrolytes;Materials Science;Organic Solvents;ReagentPlus;ReagentPlus Solvent Grade Products;Pesticide Residue Analysis (PRA) Solvents;Solvents for GC applications;Solvents for Organic Residue Analysis;Trace Analysis Reagents &;GC Solvents;Nitrile series;Nitriles;INORGANIC & ORGANIC CHEMICALS;Pre-Blended Mobile Phase Solvents;Products;Returnable Containers;Solvent Packaging Options;Acetonitrile and Acetonitrile Solutions;Amber Glass Bottles;Analytical Reagents;Analytical/Chromatography;Blends - CHROMASOLV for HPLC;Blends- CHROMASOLV for HPLC;Chromatography Reagents &;HPLC &;HPLC Grade Solvents (CHROMASOLV);HPLC/UHPLC Solvents (CHROMASOLV);Semi-Bulk Solvents;Solvent Bottles;Solvent by Application;Solvent by Type;UHPLC Solvents (CHROMASOLV);VerSA-Flow Products;3-MCPD;Adulterants;and NOGE;and Sterols;Applications;Artificial Sweeteners;Beverage Analysis;Bisphenol A (BPA);Bisphenol A in Bottled Water;Carbohydrates (sugars and saccharides) and Dietary Fiber;CHROMASOLV Gradient Grade;Edible Oils;Fats (fatty acids and triglycerides);Food &;Food Dyes;HPLC Gradient Grade Solvents (CHR;Acrylamide;Allergens;Amino Acids;BADGE;Beverages;BFDGE;CHROMASOLV Gradient;Essential Oils;ACS and Reagent Grade Solvents;Chemistry;LC-MS Blends;Mass Spectrometry (MS)&LC-MS;Spectroscopy;HPLC Solvents;Blends- CHROMASOLV LC-MSCHROMASOLV Solvents (HPLC, LC-MS);CHROMASOLV(R) LC-MSSpectroscopy;LC-MS BlendsSolvents;LC-MS Plus and Gradient;Protein Sequencing;Protein Structural Analysis;Reagents for Protein Sequencing;Analytical Chemistry;Solvents for HPLC & Spectrophotometry;Solvents for Spectrophotometry;Blends - CHROMASOLV for HPLCSemi-Bulk Solvents;Blends- CHROMASOLV for HPLCSolvents;CHROMASOLV(R) HPLC Grade SolventsSolvents;VerSA-Flow? Products;Acetonitrile and Acetonitrile SolutionsSolvent Bottles;Amber Glass Bottles;CHROMASOLV Solvents (HPLC, LC-MS);Pre-Blended Mobile Phase Solvents;Solvents;75-05-8 | | Mol File: | 75-05-8.mol |  |
| 融点 | ?45 °C (lit.) | | 沸点 | 81-82 °C (lit.) | | 比重(密度) | 0.786 g/mL at 25 °C (lit.) | | 蒸気密度 | 1.41 (vs air) | | 蒸気圧 | 72.8 mm Hg ( 20 °C) | | 屈折率 | n20/D 1.344(lit.) | | 闪点 | 48 °F | | 貯蔵温度 | Store at +5°C to +30°C. | | 溶解性 | organic solvents: soluble(lit.) | | 酸解離定数(Pka) | 25(at 25℃) | | 外見 | liquid | | 色 | <10(APHA) | | 比重 | approximate 0.78(20/20℃) | | 臭い (Odor) | Aromatic ether-like odor detectable at 40 ppm | | Relative polarity | 0.46 | | 爆発限界(explosive limit) | 3.0-17%(V) | | 臭気閾値(Odor Threshold) | 13ppm | | 水溶解度 | miscible | | 極大吸収波長 (λmax) | λ: 195 nm Amax: ≤0.12
λ: 200 nm Amax: ≤0.032
λ: 230 nm Amax: ≤0.0044
λ: 235 nm Amax: ≤0.0044
λ: 250 nm Amax: ≤0.0044
λ: 400 nm Amax: ≤0.0044 | | Merck | 14,70 | | BRN | 741857 | | Henry's Law Constant | 7.30 at 5 °C, 8.90 at 10 °C, 11.6 at 15 °C, 14.6 at 20 °C, 17.6 at 25 °C (headspace-GC, Ji and
Evans, 2007) | | 暴露限界値 | TLV-TWA 70 mg/m3 (40 ppm) (ACGIH and OSHA); STEL 105 mg/m3 (60 ppm) (ACGIH); IDLH 4000 ppm (NIOSH). | | Dielectric constant | 37.5(21℃) | | 安定性: | Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. | | LogP | -0.340 | | CAS データベース | 75-05-8(CAS DataBase Reference) | | NISTの化学物質情報 | Acetonitrile(75-05-8) | | EPAの化学物質情報 | Acetonitrile (75-05-8) |
| | アセトニトリル Usage And Synthesis |
| 外観 | 無色澄明の液体 | | 溶解性 | 水及びほとんどの有機溶媒と任意の割合で混和する。水及びエタノールに極めて溶けやすい。 | | 解説 | アセトニトリル,エーテル様臭気を有する無色の液体.融点-44.9 ℃,沸点81.6 ℃.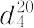 0.7828. 0.7828.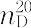 1.3442.水,メタノール,エタノールなどに可溶.アクリル系合成繊維の紡糸溶剤,ブタジエン,イソプレンなどの選択的溶剤に用いられる.また,アセトフェノン,エチルアミン,ビタミン B1 などの合成原料にも使用される. 1.3442.水,メタノール,エタノールなどに可溶.アクリル系合成繊維の紡糸溶剤,ブタジエン,イソプレンなどの選択的溶剤に用いられる.また,アセトフェノン,エチルアミン,ビタミン B1 などの合成原料にも使用される. 森北出版「化学辞典(第2版) | | 用途 | 有機合成の溶媒として、医薬、農薬、精密化学など幅広く使用されています。
| | 用途 | 汎用試薬、溶剤、有機合成原料。 | | 用途 | 環境ホルモンの分析に使用します。 | | 用途 | 高速液体クロマトグラフ分析におけるチウラム定量用の溶媒及び溶離液。 | | 用途 | 汎用試薬、高純度を要する溶剤等。 | | 用途 | 農薬?医薬?香料?染料有機合成用原料、抗生物質抽出剤、クロマト分離のキャリアー液等の抽出?分離用溶剤、カラーフィルム処理用溶剤、反応溶剤、精製溶剤、リチウム電池用有機電解液、ビタミンB1、サルファ剤の製造原料、ブタジエン抽出溶剤 | | 用途 | ビタミン B 1 等の原料、有機合成原料 | | 特長 | 1.各ロットごとにLC/MS分析適合性試験を実施2.m/z150~2,000でのノイズレベルを保証3.アルミキヤツプを採用し品質の長期安定化を実現 | | 特長 | ・本品で調製したチウラム10ppm溶液は、25℃で3日間は安定。 (保存安定性の向上)・標準液は繰り返し使用が可能となり調液操作が短縮できる。 (測定の効率化)・濃度変動に対する補正の必要がない。 (再現性の向上) | | 製造 | アセトニトリル,シアン化メチル(methyl cyanide)ともいう.アセチレンとアンモニアの反応でも合成できるが,現在の工業製品は,主としてプロペンのアンモ酸化によるアクリロニトリルの合成において副生するものが利用されている. | | 使用上の注意 | 不活性ガス封入 | | 説明 | Acetonitrile is a liquid with an etherlike odor. It is a highly
polar, volatile solvent used in many different industrial applications.
It is widely used in the pharmaceutical, photographic,
chemical, and analytical industries. It is useful as an industrial
solvent for the separation of olefins, polymers, spinning fibers,
and plastics. Other uses include the extraction and refining of
copper and by-product ammonium sulfate; used for dyeing
textiles and in coating compositions; used as a stabilizer for
chlorinated solvents; manufacture of perfumes and cosmetics;
and as a general reagent in a wide variety of chemical processes. | | 化学的特性 | Acetonitrile (methyl cyanide), CH3CN, is a colorless liquid with a sweet, ethereal odor. It is completely miscible with water and its high dielectric strength and dipole moment make it an excellent solvent for both inorganic and organic compounds including polymers. it is commonly applied to the development and manufacturing of cosmetics, pharmaceutical and agricultural products.Acetonitrile has been banned in cosmetic products in the European Economic Area (EEA) since early 2000 and acetone and ethyl are often preferred as safer for domestic use. | | 物理的性質 | Colorless liquid with an ether-like or pungent odor of vinegar. A detection odor threshold
concentration of 1,950 mg/m3 (1,161 ppmv) was experimentally determined by Dravnieks (1974).
An odor threshold concentration of 13 ppmv was reported by Nagata and Takeuchi (1990). | | 使用 | Acetonitrile is the simplest organic nitrile. It is a by-product of the manufacture of acrylonitrile, and acetonitrile has, in fact, replaced acrylonitrile. Acetonitrile has a number of uses, primarily as an extraction solvent for butadiene; as a chemical interme- diate in pesticide manufacturing; as a solvent for both inorganic and organic compounds; to remove tars, phenols, and coloring matter from petroleum hydrocarbons not soluble in acetonitrile; in the production of acrylic fi bers; in pharmaceuticals, perfumes, nitrile rubber, and acrylonitrile-butadiene-styrene (ABS) resins; in high-performance liquid and gas chro- matographic analysis; and in extraction and refi ning of copper. It is used as a starting material for the produc- tion of acetophenone, alpha-naphthalenacetic acid, thiamine, and acetamidine. | | 主な応用 | Acetonitrile is used as a solvent for polymers, spinning fibers, casting and molding plastics, and HPLC analyses; for extraction of butadiene and other olefins from hydrocarbon streams; in dyeing and coating textiles; and as a stabilizer for chlorinated solvents. It occurs in coal tar and forms as a by-product when acrylonitrile is made. Although acetonitrile is one of the more stable nitriles, it undergoes typical nitrile reactions and is used to produce many types of nitrogencontaining compounds.Acetonitrile also is used as a catalyst and as an ingredient in transitionmetal complex catalysts. | | 定義 | ChEBI: Acetonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a methyl group. It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. It is an aliphatic nitrile and a volatile organic compound. | | 調製方法 | Acetonitrile is mainly prepared by dehydration of acetamide (CH3CONH2) with
glacial acetic acid (Turner 1950) or by reacting acetic acid with ammonia at
400-500°C in the presence of a dehydration catalyst (Anon 1978). | | 一般的な説明 | A colorless limpid liquid with an aromatic odor. Flash point 42°F. Density 0.783 c / cm3. Toxic by skin absorption. Less dense than water. Vapors are denser than air. | | 空気と水の反応 | Highly flammable. Water soluble. | | 反応プロフィール | Acetonitrile decomposes when heated to produce deadly toxic hydrogen cyanide gas and oxides of nitrogen. Strongly reactive [Hawley]. May react vigorously with strong oxidizing reagents, sulfuric acid, chlorosulfonic acid, sulfur trioxide, perchlorates, nitrating reagents, and nitric acid. [Sax, 9th ed., 1996, p. 20]. Potentially explosive in contact with nitrogen-fluorine compounds (e.g., tetrafluorourea) [Fraser, G. W. et al., Chem. Comm., 1966, p. 532]. | | 健康ハザード | Acetonitrile liquid or vapor is irritating to the skin, eyes, and respiratory tract. Acetonitrile
has only a modest toxicity, but it can be metabolized in the body to hydrogen cyanide
and thiocyanate. Acetonitrile causes delayed symptoms of poisoning (several hours after
the exposure) that include, but are not limited to, salivation, nausea, vomiting, anxiety,
confusion, hyperpnea, dyspnea, respiratory distress, disturbed pulse rate, unconscious-
ness, convulsions, and coma. Cases of acetonitrile poisoning in humans (or, more strictly,
of cyanide poisoning after exposure to acetonitrile) are rare but not unknown, by inha-
lation, ingestion, and (possibly) by skin absorption. Repeated exposure to acetonitrile
may cause headache, anorexia, dizziness, weakness, and macular, papular, or vesicular
dermatitis. | | 燃焼性と爆発性 | Acetonitrile is a flammable liquid (NFPA rating = 3), and its vapor can travel a
considerable distance to an ignition source and "flash back." Acetonitrile vapor
forms explosive mixtures with air at concentrations of 4 to 16% (by volume).
Hazardous gases produced in a fire include hydrogen cyanide, carbon monoxide,
carbon dioxide, and oxides of nitrogen. Carbon dioxide or dry chemical
extinguishers should be used for acetonitrile fires. | | 工業用途 | Acetonitrile is used as a solvent both in industry and in the laboratory, as a
rodenticide, and in the denaturation of alcohol. Because of both its solvent
properties and volatility, it is useful for extracting vegetable and animal oils and
dissolving hydrocarbons, oils, and greases. Acetonitrile is used for the purification
of acetylene and artificial textile fibers, and as an antioxidant for rubber (Dequidt
et al 1974). It has also been used to extract herbicide residues from soils (Smith
1980), to remove tars and other compounds from petroleum hydrocarbons, and to
extract fatty acids from vegetable and fish liver oil. Acetonitrile is now a standard
solvent component in reversed-phase high-performance liquid chromatography. It
is the starting point for the syntheses of a number of organic compounds such as
carboxylic acids and various nitrogen derivatives (Smiley 1981). | | 安全性プロファイル | Poison by ingestion and intraperitoneal routes. Moderately toxic by several routes. An experimental teratogen. Other experimental reproductive effects. A skin and severe eye irritant. Human systemic effects by ingestion: convulsions, nausea or vomiting, and metabolic acidosis. Human respiratory system effects by inhalation. Mutation data reported. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosion Hazard: See also CYANIDE and NITRILES. When heated to decomposition it emits highly toxic fumes of CNand NOx,. Potentially explosive reaction with lanthanide perchlorates and nitrogen-fluorine compounds. Exothermic reaction with sulfuric acid at 53°C. Will react with water, steam, acids to produce toxic and flammable vapors. Incompatible with oleum, chlorosulfonic acid, perchlorates, nitrating agents, inchum, dinitrogen tetraoxide, N-fluoro compounds (e.g., perfluorourea + acetonitrile), HNO3, so3. To fight fire, use foam, Con, dry chemical | | 職業ばく露 | Acetonitrile is used as an extractant for animal and vegetable oils, as a solvent; particularly in the pharmaceutical industry, and as a chemical intermediate in pesticide manufacture; making batteries and rubber products. It is present in cigarette smoke | | 発がん性 | Under the conditions of these 2-
year inhalation studies by NTP, there was equivocal evidence
of carcinogenic activity of acetonitrile in male F344/N rats
based on marginally increased incidences of hepatocellular
adenoma and carcinoma. There was no evidence of carcinogenic
activity of acetonitrile in female F344/N rats exposed
to 100, 200, or 400 ppm. There was no evidence of carcinogenic
activity of acetonitrile in male or female B6C3F1 mice
exposed to 50, 100, or 200 ppm. Exposure to acetonitrile by
inhalation resulted in increased incidences of hepatic basophilic
foci in male rats and of squamous hyperplasia of the
forestomach in male and female mice. | | 環境運命予測 | Biological. Resting cell suspensions of the soil methylotroph Methylosinus trichosporium OB-
3b rapidly metabolized acetonitrile via oxygen insertion into the C-H bond generating the
intermediate formaldehyde cyanohydrin. The latter compound loses hydrogen cyanide yielding
formaldehyde which is then oxidized to formate (HCO2H) and bicarbonate ion (Castro et al.,
1996).
Photolytic. A rate constant of 4.94 x 10-14 cm3/molecule?sec at 24 °C was reported for the vaporphase
reaction of acetonitrile and OH radicals in air (Harris et al., 1981). Reported rate constants
for the reaction of acetonitrile and OH radicals in the atmosphere and in water are 1.90 x 10-14 and 3.70 x 10-14 cm3/molecule?sec, respectively (Kurylo and Knable, 1984). The estimated lifetime of
acetonitrile in the atmosphere is estimated to range from 6 to 17 months (Arijs and Brasseur,
1986).
Chemical/Physical. The estimated hydrolysis half-life of acetonitrile at 25 °C and pH 7 is
>150,000 yr (Ellington et al., 1988). No measurable hydrolysis was observed at 85 °C at pH
values 3.26 and 6.99. At 66.0 °C (pH 10.42) and 85.5 °C (pH 10.13), the hydrolysis half-lives
based on first-order rate constants were 32.2 and 5.5 d, respectively (Ellington et al., 1987). The
presence of hydroxide or hydronium ions facilitates hydrolysis transforming acetonitrile to the
intermediate acetamide which undergoes hydrolysis forming acetic acid and ammonia (Kollig,
1993). Acetic acid and ammonia formed react quickly forming ammonium acetate.
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 28 mg/L. The adsorbability of the carbon used was 194 mg/g carbon (Guisti et
al., 1974).
Burns with a luminous flame (Windholz et al., 1983), releasing toxic fumes of hydrogen
cyanide. | | 代謝 | Acetonitrile metabolism in dogs was demonstrated by Lang (1894), who reported that about 20% of the nitrile administered was converted to thio-cyanate in the urine, while guinea pigs metabolized acetonitrile to a greater extent (50% of dose excreted as thiocyanate). When the animals were pre-treated with ethanol, acetonitrile metabolism was induced (Tanii and Hashimoto 1986). In rats, acetone was found to potentiate acetonitrile toxicity and elevate cyanide concentrations in the blood (Freeman and Hays 1985). Baumann et al (1933) found that rabbits injected with acetonitrile excreted 27-35% of the dose as thiocyanate, while in thyroidectomized rabbits, the excretion decreased significantly (3-5% of the dose). Thiocyanate excretion was increased notably upon feeding dessicated thyroid to these animals. Hunt (1923) found that powdered sheep thyroid protected mice against acetonitrile toxicity. However, the role played by the thyroid in the detoxication of cyanide to thiocyanate is unclear. It has been suggested that the thyroid may have a role in the microsomal cleavage of cyanide from acetonitrile other than its direct effect on sulphation of cyanide to thiocyanate. | | 貯蔵 | Acetonitrile should be
used only in areas free of ignition sources, and quantities greater than 1 liter should
be stored in tightly sealed metal containers in areas separate from oxidizers. | | 輸送方法 | UN1648 Acetonitrile, Hazard Class: 3; Labels: 3-Flammable liquid | | 純化方法 | Commercial acetonitrile is a by-product of the reaction of propylene and ammonia to acrylonitrile. The following procedure that significantly reduces the levels of acrylonitrile, allyl alcohol, acetone and *benzene was used by Kiesel [Anal Chem 52 2230 1988]. Methanol (300mL) is added to 3L of acetonitrile fractionated at high reflux ratio until the boiling temperature rises from 64o to 80o, and the distillate becomes optically clear down to = 240nm. Add sodium hydride (1g) free from paraffin, to the liquid, reflux for 10minutes, and then distil rapidly until about 100mL of residue remains. Immediately pass the distillate through a column of acidic alumina, discarding the first 150mL of percolate. Add 5g of CaH2 and distil the first 50mL at a high reflux ratio. Discard this fraction, and collect the following main fraction. The best way of detecting impurities is by gas chromatography. Usual contaminants in commercial acetonitrile include H2O, acetamide, NH4OAc and NH3. Anhydrous CaSO4 and CaCl2 are inefficient drying agents. Preliminary treatment of acetonitrile with cold, saturated aqueous KOH is undesirable because of base-catalysed hydrolysis and the introduction of water. Drying by shaking with silica gel or Linde 4A molecular sieves removes most of the water in acetonitrile. Subsequent stirring with CaH2 until no further hydrogen is evolved leaves only traces of water and removes acetic acid. The acetonitrile is then fractionally distilled at high reflux, taking precaution to exclude moisture by refluxing over CaH2 [Coetzee Pure Appl Chem 13 429 1966]. Alternatively, 0.5-1% (w/v) P2O5 is often added to the distilling flask to remove most of the remaining water. Excess P2O5 should be avoided because it leads to the formation of an orange polymer. Traces of P2O5 can be removed by distilling from anhydrous K2CO3. | | Toxicity evaluation | If released to ambient air, acetonitrile will remain in the vapor
phase where it will be degraded through reaction with photochemically
produced hydroxyl radicals. The half-life of acetonitrile
in ambient air has been estimated to be about 620 days. If released
to soil, acetonitrile is expected to volatilize rapidly. Biodegradation
in soil is not expected to be a major degradation pathway. If
released to water, acetonitrile is not likely to adsorb to soil and
sediment particles. Acetonitrile is expected to be removed from
water bodies through volatilization, as the chemical hydrolysis
and bioaccumulation potential for this chemical are low. | | 不和合性 | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, chlorosulfonic acid, oleum, epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids | | 廃棄物の処理 | Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with nitrogen oxide removal from effluent gases by scrubbers or incinerators |
|