|
ChemicalBook Optimization Suppliers |
| 名前: |
Energy Chemical |
| 電話番号: |
021-021-58432009 400-005-6266 |
| 電子メール: |
sales8178@energy-chemical.com |
|
| 化学名: | テノキシカム | | 英語化学名: | Tenoxicam | | 别名: | 4-Hydroxy-2-methyl-N-pyridin-2-yl-2H-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide (Tenoxicam);MOBIFLEX;tilcotil;3-Chlorosulfonyl-2-thiophene carboxylic acid methy;Do1men;4-Hydroxy-2-methyl-N-2-pyridinyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxamidel,1-dioxide;2H-Thieno(2,3-e)-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide;4-Hydroxy-2-methyl-N-2-pyrimidinyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide | | CAS番号: | 59804-37-4 | | 分子式: | C13H11N3O4S2 | | 分子量: | 337.37 | | EINECS: | 620-500-8 | | カテゴリ情報: | Active Pharmaceutical Ingredients;Lipid signaling;Amines;Heterocycles;Sulfur & Selenium Compounds;Pharmaceutical raw material;API;TARACTAN;Tenoxicam;Intermediates & Fine Chemicals;Pharmaceuticals;Fused Ring Systems | | Mol File: | 59804-37-4.mol | 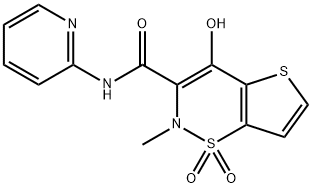 |
| 融点 | 209-2130C (dec) | | 比重(密度) | 1.4737 (rough estimate) | | 屈折率 | 1.6390 (estimate) | | 貯蔵温度 | 2-8°C | | 溶解性 | Practically insoluble in water, sparingly soluble in methylene chloride, very slightly soluble in anhydrous ethanol. It dissolves in solutions of acids and alkalis. | | 酸解離定数(Pka) | pKa1 5.3, pKa2 1.1(at 25℃) | | 色 | White to Yellow to Green | | 水溶解度 | 61.9mg/L(32 ºC) | | CAS データベース | 59804-37-4 |
| | テノキシカム Usage And Synthesis |
| 外観 | 白色~黄色~緑色粉末~結晶 | | 効能 | 抗炎症薬, シクロオキシゲナーゼ阻害薬 | | 説明 | Tenoxicam is a non-steroidal antiinflammatory agent with a profile similar to related
piroxicam and now withdrawn isoxicam (55). It is useful in the treatment of rheumatoid
arthritis, osteoarthritis and related disorders. | | 化学的特性 | Yellow Crystalline Powder | | Originator | Hoffmann-La Roche (Switzerland) | | 使用 | Tenoxicam has been used:
as a non-steroidal anti-inflammatory agent (NSAID) to study its effects on root gravitropism in Arabidopsis thaliana
as a standard in microanalysis of NSAIDs by spectrophotometry
to test its effect on surface potential andmembrane fluidity modification in phosphoglyceride monolayers | | 定義 | ChEBI: Tenoxicam is a thienothiazine-derived monocarboxylic acid amide obtained by formal condensation of the carboxy group of 4-hydroxy-2-methylthieno[2,3-e][1,2]thiazine-3-carboxylic acid 1,1-dioxide with the amino group of 2-aminopyridine. Used for the treatment of pain and inflammation in osteoarthritis and rheumatoid arthritis. It is also indicated for short term treatment of acute musculoskeletal disorders including strains, sprains and other soft-tissue injuries. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic and an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor. It is a heteroaryl hydroxy compound, a monocarboxylic acid amide, a member of pyridines and a thienothiazine. | | brand name | Tilcotil | | Biochem/physiol Actions | Tenoxicam (TX) possesses antipyretic?and analgesic effects. It elicits radical scavenging activity and has the potential to treat enkylosing spondylitis, extra-articular diseases, acute gout, and rheumatic diseases. It is also effective in treating primary dysmenorrhea, postpartum uterine contraction pain, and post-operation backaches. TX is capable of inhibiting prostaglandin synthesis. | | 臨床応用 | NSAID and analgesic | | 合成 | The reaction of methyl 3-hydroxythiophene-
2-carboxylate with PCl5 in refluxing
CCl4 gives 3-chlorothiophene-2-carboxylic
acid, which by treatment with NaHSO3
and Cu in aqueous alkaline solution at 143 ?C
in a pressure vessel is converted into 3-
sulfothiophene-2-carboxylic acid. Its esterification
with refluxing methanol affords methyl-
3-sulfothiophene-2-carboxylate, which by reaction
with refluxing SOCl2 yields methyl-
3-chlorosulfonylthiophene-2-carboxylate. The
following condensation with sarcosine ethyl
ester in hot CHCl3 gives 3-(N-ethoxycarbonylmethyl-
N-methylsulfamoyl)thiophene-2-carboxylate,
which is cyclized by treatment with
sodium methoxide in refluxing methanol affording
3-ethoxycarbonyl-4-hydroxy-2-methyl-2Hthieno--1,2-thiazine 1,1-dioxide. Finally
this compound is condensed with 2-aminopyridine
in refluxing toluene | | 薬物相互作用 | Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. | | 代謝 | Metabolised in the liver via cytochrome P450 2C9 to
several pharmacologically inactive metabolites (mainly
5'-hydroxy-tenoxicam).
Metabolites are excreted mainly in the urine; there is
some biliary excretion of glucuronide conjugates of the
metabolites. |
|