|
ChemicalBook Optimization Suppliers |
| 名前: |
LGM Pharma |
| 電話番号: |
1-(800)-881-8210 |
| 電子メール: |
inquiries@lgmpharma.com |
|
| 化学名: | ホミノベン | | 英語化学名: | Fominoben | | 别名: | FOMINOBEN;3'-Chloro-2'-(N-methyl-N-((morpholinocarbonyl)methyl)aminomethyl)benzanilide;N-[3-Chloro-2-[[methyl-(2-morpholin-4-yl-2-oxoethyl)amino]methyl]phenyl]benzamide;3'-Chloro-a-[methyl[(morpholinocarbonyl)methyl]amino]-o-benzotoluidide;Benzamide, N-[3-chloro-2-[[methyl[2-(4-morpholinyl)-2-oxoethyl]amino]methyl]phenyl]-;N-(2-Benzoylamino-6-chlorobenzyl)-N-methylglycine morpholide;Terion;Fominoben USP/EP/BP | | CAS番号: | 18053-31-1 | | 分子式: | C21H24ClN3O3 | | 分子量: | 401.89 | | EINECS: | 241-964-4 | | カテゴリ情報: | | | Mol File: | 18053-31-1.mol | 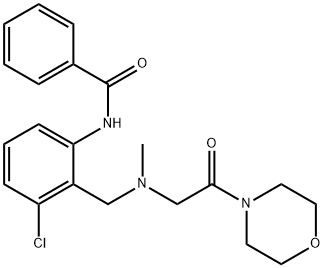 |
| | ホミノベン Usage And Synthesis |
| 効能 | 鎮咳薬 | | Originator | Noleptan,Thomae,W. Germany,1973 | | 定義 | ChEBI: Fominoben is a member of benzamides. | | Manufacturing Process | (a) A mixture consisting of 9.75 g of 6-chloro-2-dibenzoylamino-benzyl
bromide, 2.34 g of sarcosine methyl ester, 3.18 ml of triethylamine and 250
ml of chloroform was refluxed for five hours. Thereafter, an addition 0.5 g of
sarcosine methyl ester was added, and the mixture was again refluxed for five
hours. Subsequently, the chloroform was evaporated in vacuo, the residue was
taken up in ethylacetate, the insoluble matter was separated by filtration, and
the filtrate was again evaporated in vacuo. The residual oil was dissolved in
methanol, the solution was admixed with 25 ml of 2 N sodium hydroxide, and
the mixture was allowed to stand overnight at about 20°C. Thereafter, the
methanol was evaporated in vacuo, and the residual aqueous solution was
adjusted to pH 2 with 2 N hydrochloric acid, then extracted with ethyl acetate
and then adjusted to pH 6 with 2N sodium hydroxide. The crystalline product
precipitated thereby was collected by vacuum filtration and recrystallized from
water, yielding N-(2-benzoylamino-6-chloro-benzyl)-N-methyl-glycine, MP
150°C to 152°C.
(b) 80.7 g of N-(2-benzoylamino-6-chlorobenzyl)-N-methyl-glycine and 38 ml
of triethylamine were dissolved in 1 liter of dry chloroform. While stirring the
resulting solution at -15°C to -5°C, 23.4 ml of ethyl chloroformate were
rapidly added dropwise, and the mixture was stirred for 40 minutes more at -
15°C to -5°C. Thereafter, 50 ml of morpholine were added all at once, and the
mixture was allowed to stand at 20°C for 20 hours. Subsequently, the
chloroformic reaction solution was washed three times with brine, dried over
magnesium sulfate and evaporated in vacuo, and the oily residue was taken
up in ether, whereupon it crystallized. The crystalline product was
recrystallized from methanol, yielding N-(2-benzoyl-amino-6-chloro-benzyl)-N�methyl-glycine-morpholide, MP 122.5°C to 123°C.
The product was dissolved in isopropanol, and the solution was acidified with
anhydrous hydrochloric acid, yielding the hydrochloride, MP 206°C to 208°C
(decomp.). | | Therapeutic Function | Antitussive, Respiratory stimulant Fominoben hydrochloride |
|