|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アファチニブマレイン酸塩 | | 英語化学名: | Afatinib dimaleate | | 别名: | BIBW2992 DiMaleate;BIBW2992-MA2;Afatinib (diMaleate), BIBW2992;2-ButenaMide, N-[4-[(3-chloro-4-fluorophenyl)aMino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(diMethylaMino)-, (2E)-, (2Z)-2-butenedioate (1:2);(2E)-N-(4-[(3-chloro-4-fluorophenyl)aMino]-7-{[(3S);Afatinib double Maleate;Afatinib (BIBW2992) Dimaleate;N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-(2E)-2-butenamide,(2Z)-2-butenedioate (1:2) | | CAS番号: | 850140-73-7 | | 分子式: | C28H29ClFN5O7 | | 分子量: | 602.02 | | EINECS: | 810-416-1 | | カテゴリ情報: | Inhibitors;tyrosine kinase receptor inhibitor;850140-73-7 | | Mol File: | 850140-73-7.mol | 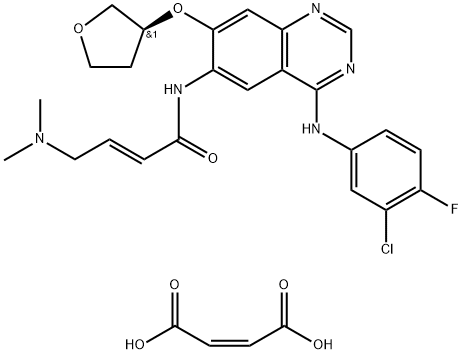 |
| 融点 | >237oC (dec.) | | 貯蔵温度 | Refrigerator | | 溶解性 | DMSO (Slightly), Methanol (Slightly) | | 外見 | Solid | | 色 | White to Pale Yellow | | InChIKey | LIENDGDDWJRJLO-LBXKZPGENA-N | | SMILES | C(/C(=O)O)=C/C(=O)O.N(C1C=CC(F)=C(Cl)C=1)C1=NC=NC2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC1=2 |&1:25,r| |
| | アファチニブマレイン酸塩 Usage And Synthesis |
| 効能 | 抗悪性腫瘍薬, 受容体チロシンキナーゼ阻害薬 | | 商品名 | ジオトリフ (日本ベーリンガーインゲルハイム) | | 説明 | Afatinib dimaleate was approved by the U.S. Food and Drug
Administration (FDA) in 2013 for the treatment of non-small cell
lung cancer (NSCLC). Specifically, it was approved for patients
presenting with metastatic NSCLC tumors which contain epidermal
growth factor receptor (EGFR) exon deletions or exon 21
mutations. Afatinib dimaleate is a covalent inhibitor of ErbB tyrosine
kinases (tyk), which downregulates ErbB signaling by irreversible
binding of EGFR tyk binding sites. While no
manufacturing route has been disclosed to date, the most scalable
published route likely derives from two Boehringer Ingelheim
patents. | | 使用 | Afatinib Dimaleate is a salt of Afatinib {BIBW 2992), an aminocrotonylamino-substituted quinazoline derivative used for treating cancer and diseases of the respiratory tract, lungs, gastrointestinal tract, bile duct, and gallbladder. An anilino-quinazoline that irreversibly inhibits EGFR and HER2 kinase activity. | | 定義 | ChEBI: Afatinib dimaleate is a maleate salt obtained by combining afatinib with two molar equivalents of maleic acid. Used for the first-line treatment of patients with metastatic non-small cell lung cancer. It has a role as a tyrosine kinase inhibitor and an antineoplastic agent. It contains an afatinib. | | 一般的な説明 | Afatinib dimaleate is the dimaleate form of afatinib. It is a tyrosine kinase inhibitor of epidermal growth factor receptors (ERBB RECEPTORS) and an anti-angiogenic agent. Afatinib dimaleate has anti-tumour activity and is mainly used for the treatment of patients with metastatic non-small cell lung cancer (which has metastasised or spread to other parts of the body), or certain tumours with mutations in the EGFR gene and patients whose disease has worsened after platinum-based chemotherapy. It is also used for the treatment of esophageal squamous cell carcinoma (ESCC) and gastric cancer. | | 生物活性 | Afatinib dimaleate is an orally bioavailable and irreversible dual-specific inhibitor of the ErbB family (EGFR and HER2). IC50 are 0.4, 0.5, 10, 14, 1 nM for EGFRL858R, EGFRwt, EGFR L858R/T790M, ErbB2 (HER2) and ErbB4 (HER4), respectively. Afatinib dimaleate is available for cancer (esophageal squamous cell carcinoma (ESCC), non-small cell lung cancer (NSCLC) and gastric cancer) studies. | | 合成 | Nitroquinazolinone (6), which is commercially available, was
first chlorinated with phosphorous oxychloride (POCl3) followed
by treatment with commercial 3-chloro-4-fluoroaniline (7) to
afford SNAr adduct 8 in 90% yield over two steps. Sulfonylation to afford 9 (86%) and subsequent displacement with (S)-tetrahydrofuran-
3-ol gave 10 in 90% yield. Raney¨CNickel reduction
of the nitro group delivered 11 in 97% yield, which set the stage
for the final side-chain functionalization. 2-(Diethoxyphosphoryl)
acetic acid and N,N0-carbonyldiimidazole (CDI) were pre-mixed
and added to aniline 11 to afford 12 in 70% isolated yield. Next, a
Horner¨CWadsworth¨CEmmons homologation gave the (E)-olefin
13 in quantitative yield, followed by maleate salt formation
(92%) to deliver the final API. The final five steps of this synthesis
have been successfully demonstrated on multi-kilogram scale. 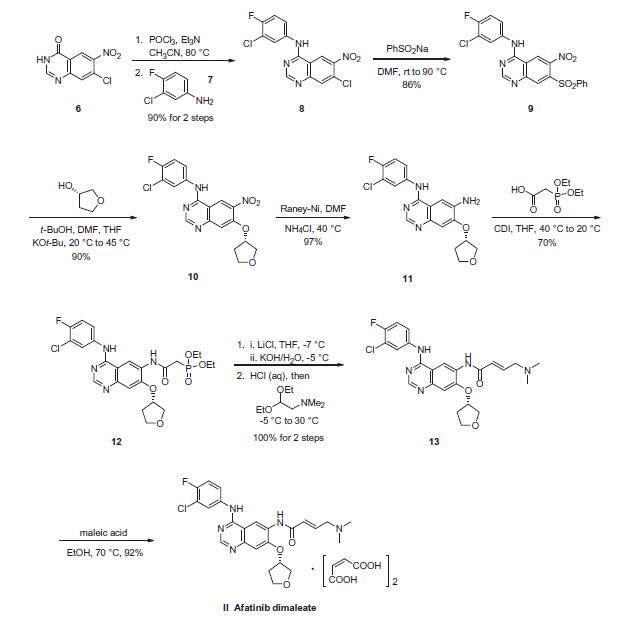
| | 貯蔵 | Store at -20°C |
|