|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 炭酸カルシウム(II)(1:1) | | 英語化学名: | CALCIUM CARBONATE | | 别名: | PARIS WHITE;CALCIUM CARBONATE 325;WHITING;lime stones;CHALK, PRECIPITATED;ENGLISH WHITE;KALKSPAR;ICELAND SPAR | | CAS番号: | 1317-65-3 | | 分子式: | CCaO3 | | 分子量: | 100.09 | | EINECS: | 215-279-6 | | カテゴリ情報: | | | Mol File: | 1317-65-3.mol | 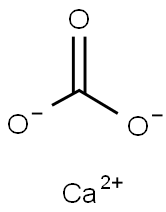 |
| | 炭酸カルシウム(II)(1:1) Usage And Synthesis |
| 定義 | 本品は、主として炭酸カルシウムからなる鉱物である。 | | 解説 | あられ石,CaCO3よりなる鉱物.空間群 Pmcnの斜方晶系.格子定数 a0 = 0.496,b0 = 0.796,c0 = 0.574 nm.単位格子中4個の基本組成を含む.硬度3.5~4.密度2.947 g cm-3.紫外線,X線,電子線により蛍光を発する.Caの一部はわずかにSr,Pb,Znで置換される.方解石と多形の関係にあるが不安定である.火成岩の晶洞,あるいは低温の地表,温泉地帯に産出する. | | 化粧品の成分用途 | 皮膚コンディショニング剤 | | 説明 | Calcite is one of the most common minerals on the face of
the earth, comprising about 4% by weight of the earth’s
crust and is formed in many different geological environments.
Calcite can form rocks of considerable mass
and constitutes a significant part of all three major
rock classification types. It forms oolitic, fossiliferous
and massive limestones in sedimentary environments
and even serves as the internal cement for many sandstones
and shales.Calcite is even a major component in the igneous rock called carbonatite and forms the
major portion of many hydrothermal veins. Not necessarily
a variety of calcite, cave formations are certainly
a unique aspect of calcite’s story. | | 化学的特性 | Calcium carbonate is a white, odorless
powder, or crystalline solid. | | 化学的特性 | Ground limestone consists essentially of calcium carbonate. It is
obtained by crushing, grinding, and classifying naturally occurring
limestone benefited by flotation and/or air classification. It is pro duced as a fine, white to off-white, microcrystalline powder. It is
odorless and tasteless and is stable in air. It is practically insoluble
in water and in alcohol. The presence of any ammonium salt or
carbon dioxide increases its solubility in water, but the presence
of any alkali hydroxide reduces the solubility. | | 使用 | Manufacture of quicklime, Portland
cement, and paints. United States Pharmacopeia
(USP) grades are used in dentifrices,
cosmetics, food, and pharmaceuticals such as
antacids. | | 使用 | Source of lime; neutralizing agent, filler, and
extender in rubber, plastics, paints; opacifying agent
in paper; fortification of bread; putty; tooth pow-
ders; antacid; whitewash; Portland cement; sulfur
dioxide removal from stack gases; metallurgical
flux; analytical chemistry; carbon dioxide gener-
ation (laboratory). | | 使用 | Calcite is the primary
mineral component in cave formations. Stalactites and
stalagmites, cave veils, cave pearls, “soda straws” and
the many other different cave formations that millions
of visitors to underground caverns enjoy are made of
calcite. | | 製造方法 | The calcite present is derived mostly from the remains of organisms
such as clams, brachiopods, bryozoa, crinoids and
corals. These animals live on the bottom of the sea and
when they die their shells accumulate into piles of shelly debris. This debris can then form beds of limestone.
Some limestones may have been derived from nonbiogenic
calcite formation. | | 定義 | Aragonite: An
anhydrous mineral form of calcium carbonate,
CaCO3, which occurs associated
with limestone and in some metamorphic
rocks. It is also the main ingredient of
pearls. It is not as stable as calcite, into
which it may change over time.
Calcite:A mineral form of calcium
carbonate occurring in limestone,
chalk, and marble.
Charlk: A natural form of calcium carbonate
(CaCO3) formed originally by marine
organisms. (Blackboard chalk is calcium
sulfate, CaSO4.). | | 一般的な説明 | Odorless, white to tan powder. | | 反応プロフィール | CALCIUM CARBONATE has generally low chemical reactivity. Is non-combustible. Decomposes at high temperature (825°C) to give gaseous carbon dioxide and calcium oxide (quicklime). Incompatible with acids, alum, ammonium salts, fluorine, magnesium. Reacts with acids and acidic salts to generate gaseous carbon dioxide with effervescence (bubbling). The reaction is rapid and exothermic with concentrated solutions of acids. The efferversence can create extensive foaming. Ignites on contact with fluorine. | | 危険性 | A nuisance particulate dust. | | 健康ハザード | Calcium carbonate is considered
to be a nuisance dust.
Although no adverse effects have been
reported in the literature among workers
exposed to calcium carbonate, high concentrations
of the dust would be expected to act as a
physical irritant to the eyes and skin.1 Fourteen
British workers exposed to heavy calcium carbonate
concentrations for 12–35 years showed
no trace abnormalities due to dust or any
clinical sign of pneumoconiosis or chronic
bronchitis on X ray.2 Long exposure to high
dust concentrations of pure calcium carbonate
(quartz content less than 1.1%) did not result
in lung fibrosis. | | 农业用途 | Limestone or dolomite, used in agriculture for liming the soil, is ground to a fineness to ensure that 50% of the particles pass through a 1.70mm Indian Standards (IS) sieve, and 50% is retained on a 150micron IS sieve. | | 安全性プロファイル | A nuisance dust. An
eye and skin irritant. Igmtes on contact with
F2. Incompatible with acids, ammonium
salts, (Mg + H2). Calcium carbonate is a
common air contaminant. See also
CALCIUM COMPOUNDS. | | 職業ばく露 | Calcium carbonate is used as a source
of lime; neutralizing agent; manufacturing or rubber, plastics, paint and coatings; sealants, paper, dentifrices, ceramics, putty, polishes and cleaners, insecticides, inks and
cosmetics; whitewash; Portland cement; antacids; analytical
chemistry, and others | | 不和合性 | Calcium carbonate decomposes in high
temperature forming carbon dioxide and corrosive
materials | | 廃棄物の処理 | Landfills. It is the responsibility of chemical waste generators to determine if a discarded
chemical is classified as a hazardous waste. See 40 CFR
Parts 261.3 for United States Environmental Protection
Agency guidelines for the classification determination. In
addition, in order to ensure complete and accurate classification, waste generators must consult state and local hazardous waste regulations. |
|