|
ChemicalBook Optimization Suppliers |
|
| 融点 | 220 °C (dec.) (lit.) | | 沸点 | 582.1±60.0 °C(Predicted) | | 比重(密度) | 1.606 | | 屈折率 | 1.6580 (estimate) | | 闪点 | 11 °C | | 貯蔵温度 | 2-8°C | | 溶解性 | Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride. It dissolves in dilute solutions of alkali hydroxides. | | 酸解離定数(Pka) | pKa 3.8 (Uncertain) | | 外見 | powder | | 色 | White to Off-White | | 水溶解度 | Soluble in acetone, DMF or methanol. Slightly soluble in water | | Merck | 14,4309 | | BCS Class | 2 (CLogP), 4
(LogP) | | 安定性: | Stable, but light sensitive, air sensitive and hygroscopic. Incompatible with strong oxidizing agents. | | InChIKey | ZZUFCTLCJUWOSV-UHFFFAOYSA-N | | CAS データベース | 54-31-9(CAS DataBase Reference) | | IARC | 3 (Vol. 50) 1990 | | EPAの化学物質情報 | Furosemide (54-31-9) |
| | フロセミド Usage And Synthesis |
| 解説 | 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.C12H11ClN2O5S(330.74).2,4-ジクロロ安息香酸にアミノスルホニル基を導入し,2-アミノメチルフランと縮合させると得られる.白色の結晶.分解点205 ℃.フロセミドは,水に不溶,メタノール,アセトンに可溶,DMFに易溶.高血圧症,うっ血性心不全治療薬として使用される.また,降圧利尿剤として尿路結石排出促進に用いられる.LD50 3000 mg/kg(ラット,経口).森北出版「化学辞典(第2版) | | 用途 | フロセミド (Furosemide)は、心不全、肝硬変、腎疾患(英語版)による浮腫の治療に用いられるループ利尿薬の一つである。降圧を目的とした処方も行われる。 | | 用途 | 利尿剤。フロセミドは,腎臓のヘンレ係蹄でのナトリウム再吸収を抑制する,すなわち尿の濃縮,希釈機構を抑制することにより,血漿とほぼ同じ浸透圧の尿を生成する。また,遠位尿細管にナトリウムが流入するため,カリウムの排泄が促進される。ネフローゼ症候群,慢性腎不全に用いられる。副作用としては低カリウム血症,代謝性アルカロージス,聴力障害,高尿酸血症などを起すおそれがある。 | | 効能 | 利尿薬, Na-K-Cl共輸送体阻害薬 | | 商品名 | オイテンシン (サノフィ); ラシックス (サノフィ); ラシックス (サノフィ); ラシックス (サノフィ) | | 説明 | Furosemide (Item No. 26298) is an analytical reference standard categorized as a diuretic. Formulations containing diuretics, including furosemide, have been misused in sports for weight reduction and as masking agents in humans and to prevent exercise-induced pulmonary hemorrhage in racehorses. This product is intended for use in analytical forensic applications. This product is also available as a general research tool . | | 化学的特性 | white to light yellow crystal powde | | Originator | Lasix,Hoechst,W. Germany,1964 | | 使用 | An inhibitor of NKCC and a GABAA receptor antagonist. | | 使用 | This compound belongs to the aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. | | 使用 | diuretic, antihypertensive | | 使用 | Furosemide inhibits ion co-transport in the kidney. Furosemide is used as a diuretic. | | 定義 | A benzoic-sulfonamide-furan. It is a diuretic with
fast onset and short duration and anti-hypertensive
agent. | | Manufacturing Process | 10.8 grams of 3-sulfamyl-4,6-dichlorobenzoic acid (0.04 mol) and 11.7 grams
of furfurylamine (0.12 mol) are heated in 30 cc of diethyleneglycol�dimethylether for 6 hours under reflux. When pouring the reaction mixture
into 300 cc of 1 N hydrochloric acid, the reaction product is immediately
separated off in the form of crystals. The light-yellow crude product is purified
by dissolving it in 100 cc of warm 1 N sodium bicarbonate solution,
precipitation by means of hydrochloric acid and subsequent recrystallization
from ethanol/water, with addition of charcoal. Colorless prisms are obtained
which decompose at 206°C while adopting a brown coloration, and with
evolution of gas. | | brand name | Lasix (Sanofi Aventis). | | Therapeutic Function | Diuretic | | 一般的な説明 | Odorless white to slightly yellow crystalline powder. A diuretic drug. Almost tasteless. | | 空気と水の反応 | Light sensitive. Air sensitive. Slightly soluble in water. | | 反応プロフィール | Furosemide may undergo hydrolysis at sufficiently low pH. The pH of aqueous solutions should be maintained in the basic range to prevent hydrolysis. Alcohol has been shown to improve the stability of Furosemide. Incompatible with strong oxidizing agents . | | 危険性 | Poison; moderately toxic; teratogen; questionable carcinogen; mutagen. | | 火災危険 | Flash point data for Furosemide are not available; however, Furosemide is probably combustible. | | 生物活性 | Loop diuretic that inhibits the Na + /2Cl - /K + (NKCC) cotransporter. Also acts as a non-competitive antagonist at GABA A receptors with ~ 100-fold greater selectivity for α 6-containing receptors than α 1-containing receptors. | | Biochem/physiol Actions | Inhibits ion co-transport in the kidney. | | 作用機序 | Furosemide is a highly effective and quick-acting diuretic whose action, like all of the
examined loop diuretics, is associated with blocking reabsorption of ions in the ascending
bend of Henle’s loop. It is used for edema syndrome of various origins, edema of the lungs
and brain, chronic renal insufficiency, some forms of hypertonic crises, and poisoning by
barbiturates and other compounds excreted mainly with urine. | | 臨床応用 | Furosemide has a saluretic effect 8- to 10-fold that of the thiazide diuretics; however, it has a shorter duration of action (~6–8 hours). Furosemide causes a marked
excretion of sodium, chloride, potassium, calcium, magnesium, and bicarbonate ions, with as much as 25% of the filtered load of sodium excreted in response to initial
treatment. It is effective for the treatment of edemas connected with cardiac, hepatic, and renal sites. Because it lowers the blood pressure similar to the thiazide
derivatives, one of its uses is in the treatment of hypertension. | | 副作用 | Clinical toxicity of furosemide and other loop diuretics primarily involves abnormalities of fluid and electrolyte balance. As with the thiazide diuretics, hypokalemia is an
important adverse effect that can be prevented or treated with potassium supplements or coadministration of potassium-sparing diuretics. Increased calcium ion excretion
can be a problem for postmenopausal osteopenic women, and furosemide generally should not be used in these individuals. Hyperuricemia, glucose intolerance,
increased serum lipid levels, ototoxicity, and gastrointestinal side effects might be observed as well. Hypersensitivity reactions also are possible with furosemide (a
sulfonamide-based drug), and cross-reactivity with other sulfonamide containing drugs is possible. | | 安全性プロファイル | Poison by intravenous
route. Moderately toxic by ingestion and
intraperitoneal routes. Human systemic
effects by intravenous route: change in the
sensitivity of the ear to sound, tinnitus,
unspecified effects on the heart, constriction
of the arteries, a decrease in urine volume,
interstitial nephritis, metabolic alkalosis,
pulse rate decrease, fall in blood pressure.
Ingestion can damage the liver.
Experimental teratogenic and reproductive
effects. Questionable carcinogen with
experimental carcinogenic effects. Human
mutation data reported. When heated to
decomposition it emits very toxic fumes of
Cl-, NOx, and SOx. | | 合成 | Furosemide, 4-chloro-N-furfuryl-5-sulfamoylanthranylic acid (21.4.11), is
synthesized in a relatively simple manner from 2,4-dichlorobenzoic acid, which is converted
into 5-aminosulfonyl-4,6-dichlorobenzoic acid (21.4.10) during subsequent reaction
with chlorosulfonic acid and ammonia. Reacting this with furfurylamine gives
furosemide (21.4.11) . 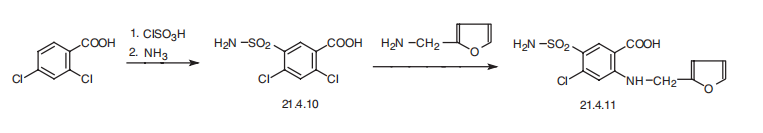
| | Veterinary Drugs and Treatments | Furosemide is used for its diuretic activity in all species. It is used
in small animals
for the treatment of congestive cardiomyopathy,
pulmonary edema, hypercalcuric nephropathy, uremia, as adjunctive
therapy in hyperkalemia and, occasionally, as an antihypertensive
agent. In cattle,
it is approved for use for the treatment of
post-parturient udder edema. It has been used to help prevent or
reduce epistaxis (exercise-induced pulmonary hemorrhage; EIPH)
in racehorses. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Analgesics: increased risk of nephrotoxicity with
NSAIDs; antagonism of diuretic effect with
NSAIDs.
Anti-arrhythmics: risk of cardiac toxicity with
anti-arrhythmics if hypokalaemia occurs; effects of
lidocaine and mexiletine antagonised.
Antibacterials: increased risk of ototoxicity with
aminoglycosides, polymyxins and vancomycin; avoid
with lymecycline.
Antidepressants: increased risk of hypokalaemia with
reboxetine; enhanced hypotensive effect with MAOIs;
increased risk of postural hypotension with tricyclics.
Antiepileptics: increased risk of hyponatraemia with
carbamazepine; effects antagonised by phenytoin.
Antifungals: increased risk of hypokalaemia with
amphotericin.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect
with alpha-blockers; increased risk of ventricular
arrhythmias with sotalol if hypokalaemia occurs.
Antipsychotics: increased risk of ventricular
arrhythmias with amisulpride or pimozide (avoid
with pimozide) if hypokalaemia occurs; enhanced
hypotensive effect with phenothiazines.
Atomoxetine: hypokalaemia increases risk of
ventricular arrhythmias.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: variable reports of increased
nephrotoxicity, ototoxicity and hepatotoxicity.
Cytotoxics: concentration of furosemide increased
by dasabuvir, ombitasvir and paritaprevir - reduce
furosemide dose; increased risk of ventricular
arrhythmias due to hypokalaemia with arsenic
trioxide; increased risk of nephrotoxicity and
ototoxicity with platinum compounds.
Lithium: risk of toxicity. | | 代謝 | Little biotransformation of furosemide takes place. It
is mainly eliminated via the kidneys (80-90%); a small
fraction of the dose undergoes biliary elimination and
10-15% of the activity can be recovered from the faeces. | | 貯蔵 | Store at RT | | 参考文献 | [1]. hochman dw. the extracellular space and epileptic activity in the adult brain: explaining the antiepileptic effects of furosemide and bumetanide. epilepsia, 2012, 53 suppl 1: 18-25.
[2]. chen h, sun d. the role of na-k-cl co-transporter in cerebral ischemia. neurol res, 2005, 27(3): 280-286.
[3]. prandota j. furosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. am j ther, 2002, 9(4): 317-328. |
|