(S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS manufacturers
|
| | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS Basic information | | Reaction |
| Product Name: | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS | | Synonyms: | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS;(S)-(+)-4,12-Bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane,min.93%CTH-(S)-3,5-xylyl-PHANEPHOS;(S)-xylyl-PHANEPHos, (S)-5,11-Bis(3,5-xylylphosphino)tricyclo[8.2.24,7]hexadeca-hexaene;S-Xylyl-Phanephos;Tricyclo[8.2.2.2(4,7)]hexadeca-4,6,10,12,13,15-hexaene-5,11-diylbis[bis(3,5-dimethylphenyl)phosphine] stereoisomer;(S)-(+)-4,12-Bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane, Min. 97% CTH-(S)-3,5-xylyl-PHANEPHOS;(S)-XylPhanephos;(S)-4,12-Bis(1,1-bis(3,5-dimethylphenyl)phosphine)-[2.2]paracyclophane | | CAS: | 325168-88-5 | | MF: | C48H50P2 | | MW: | 688.86 | | EINECS: | | | Product Categories: | Chiral Phosphine;PHANEPhos Series;organophosphine ligand | | Mol File: | 325168-88-5.mol | ![(S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS Structure](CAS/GIF/325168-88-5.gif) |
| | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS Chemical Properties |
| Melting point | 234-238 °C | | Boiling point | 785.2±60.0 °C(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | form | Powder | | color | white | | Optical Rotation | [α]/D +61.0°, c = 0.1 in ethanol |
| Hazard Codes | Xi | | Risk Statements | 36/38 | | Safety Statements | 26 | | WGK Germany | 3 |
| | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS Usage And Synthesis |
| Reaction |
- Chiral ligand employed in the enantioselective hydrogenation of various ketones.
- Chiral ligand employed in the enantioselective hydroxycarbonylation and alkoxycarbonylation of alkenes.
- Chiral ligand employed in the enantioselective [2+2] cycloaddition of oxabicyclic alkenes with terminal alkynes.
- Chiral ligand employed in the gold-catalyzed enantioselective Cope rearrangement of achiral 1,5-dienes.
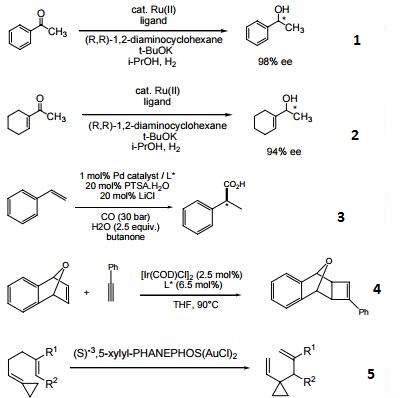
| | Uses | Efficient ligand for asymmetric hydrogenation of dehydroamino acids, methyl esters and ketones. |
| | (S)-(+)-4,12-BIS(DI(3,5-XYLYL)PHOSPHINO)-[2.2]-PARACYCLOPHANE ,CTH-(S)-3,5-XYLYL-PHANEPHOS Preparation Products And Raw materials |
|