| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-87576359 15353716720 |
| Email: |
1039@dideu.com |
| Products Intro: |
Product Name:URANIUM CHLORIDE
CAS:10026-10-5
Purity:95% Package:25kg
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | URANIUM CHLORIDE Basic information |
| Product Name: | URANIUM CHLORIDE | | Synonyms: | URANIUM TETRACHLORIDE;URANIUM CHLORIDE;UCl4;Uranium chloride (UCl4);Uranium(IV) chloride;uranium(iv)chloride;uraniumchloride(ucl4);URANIUM TETRACHLORIDE ANHYDROUS 99% | | CAS: | 10026-10-5 | | MF: | Cl4U | | MW: | 379.84 | | EINECS: | 2330577 | | Product Categories: | | | Mol File: | 10026-10-5.mol | 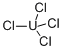 |
| | URANIUM CHLORIDE Chemical Properties |
| Melting point | 590° | | Boiling point | bp760 791° | | density | d425 4.725 | | solubility | reacts with H2O; soluble in ethanol | | form | green octahedral crystals | | color | green octahedral crystals, crystalline | | Water Solubility | very soluble H2O, with decomposition [MER06] | | EPA Substance Registry System | Uranium chloride (UCl4) (10026-10-5) |
| | URANIUM CHLORIDE Usage And Synthesis |
| Chemical Properties | dark green octahedral crystal(s); oxidizes in air; decomposes in water; enthalpy of fusion 45.00 kJ/mol; prepared by the reaction UO3+3CCl3CCl=CCl2→UCl4+Cl2+3CCl2=CClOCl [KIR83] [CRC10] [MER06] | | Production Methods | A bomb tube of ~ 1.5 cm I.D. is charged with 5.6 g of pure UOS and 10 ml of SOCI2 (freshly distilled in a vacuum); these are then thoroughly mixed. The sealed tube is heated for 7 days at 200 °C; the time required for completion of the reaction may be somewhat reduced if the tube is briefly cooled at regular intervals and the SO2 formed is permitted to escape. The product consists of UCl4, partially dissolved in SOCl2 and partially present as green crystals. The lower part of the bomb tube is cut off, and the entire contents are rinsed rapidly with some SOCl2 into a 100 ml ground-joint flask and distilled under reduced pressure (e.g., 140 mm) in a stream of dry N2 (or CO2) to remove the SOCl2. Finally, the brown adduct of SOCl2 and UCl4 is decomposed by heating at about 150°C until a pure green residue of UCl4 is obtained (half an hour is required). Yield: about 7.5 g (95%). | | Safety Profile | Probably a poison.
When heated to decomposition it emits
toxic fumes of Cl-. See also URANIUM. |
| | URANIUM CHLORIDE Preparation Products And Raw materials |
|