| Company Name: |
Hangzhou Huarong Pharm Co., Ltd. |
| Tel: |
571-86758373 +8613588754946 |
| Email: |
sales@huarongpharm.com |
| Products Intro: |
Product Name:methyl (41S,13aS)-13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
CAS:4880-92-6
Purity:0.99 Package:10MG,50MG,100MG,1G
|
|
|
|
|
|
| | apovincamine Basic information |
| Product Name: | apovincamine | | Synonyms: | Methyl (3alpha,16alpha)-eburnamenine-14-carboxylate;apovincamine;Methyl (13aS,13bS)-13a-ethyl-2,3,5,6,13a,13b-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate;methyl (41S,13aS)-13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate;(3α,16α)-Eburnamenine-14-carboxylic acid methyl ester;Apo-14,15-dehydrovincamine;Apovincaminic acid methyl;(+)-trans-Cavinton | | CAS: | 4880-92-6 | | MF: | C21H24N2O2 | | MW: | 336.43 | | EINECS: | 225-491-0 | | Product Categories: | | | Mol File: | 4880-92-6.mol | 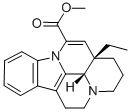 |
| | apovincamine Chemical Properties |
| Melting point | 160-162℃ | | Boiling point | 405.7±45.0 °C(Predicted) | | density | 1.30±0.1 g/cm3(Predicted) | | storage temp. | Refrigerator | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 7.82±0.60(Predicted) | | color | Off-White to Pale Grey |
| | apovincamine Usage And Synthesis |
| Originator | Apovincamine,GC Promochem | | Uses | cis-Apovincamine (Vinpocetine USP Related Compound B), is a vinca alkaloid and a chemical precursor of Vinpocetine (V332500), a derivative of Vincamine with vasodilating activity. Vasodilator (cerebral). | | Definition | ChEBI: Apovincamine is an alkaloid. | | Manufacturing Process | Apovincamine is semisynthethetic derivative of (+)-vincamine. Vincamine is
alkaloid of Vinca minor. Alcoloid of Tabernaemontana rigida (Apocynaceae)
also is used as raw material for preparing of apovincamine. (+)- Vincamine
was transformed into oxime ester by reaction with NaNO2 in acetic acid at 0°C
-(+/-)-methyl-3-((12bR)-1-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-
a]quinolizin-1-yl)-2-(hydroxyimino)propanoate, which was resolved on the
isomers with dibenzoyl D-tartratic acid. (-)-Methyl 3-((12bR)-1-ethyl-
1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-1-yl)-2-
(hydroxyimino)propanoate was re-crystallized from methanol to afford (-)-
methyloxime ester, MP: 195°C.
2 g of above oxime methyl ester was heated in mixture of methanol (37.5 ml)
and conc. H2SO4 (13.5 ml) on water bath for 1 hour. The solution was poured
into ice-water (80 ml), basified with conc. NH4OH to pH 9, and extracted with
CH2Cl2 (3x20 ml). The combined extracts were dried (MgSO4), filtered,
evaporated in vacuum and the residue was re-crystallized from MeOH (5 ml)
to yield 1.32 g (72.5%) of (+)-apovincamine, MP: 160°-162°C. | | Therapeutic Function | Vasodilator |
| | apovincamine Preparation Products And Raw materials |
|
|
Tag:apovincamine(4880-92-6)
Related Product Information
|
|
Vinpocetine
ApovincaMinic Acid Ethyl Ester N-Oxide
methyl (41R,12R,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
methyl (3alpha,14alpha,16alpha)-14,15-dihydro-14-hydroxyeburnamenine-14-carboxylate
(41S,12S,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylic acid
methyl (41R,12S,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
methyl (41S,12S,13aS)-13a-ethyl-12-hydroxy-8-methoxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
ethyl (41R,13aS)-13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
(+)-(14β)-Dihydrovinpocetine
(-)-DihydroapovincaMinic Acid Ethyl Ester
ethyl (41S,12R,13aS)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
ethyl (41S,13aR)-13a-ethyl-2,3,41,5,6,13a-hexahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
methyl (41R,12R,13aR)-13a-ethyl-12-hydroxy-2,3,41,5,6,12,13,13a-octahydro-1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12-carboxylate
16,17-Dihydroapovincamine
sodium (41S,13aS)-13a-ethyl-2,3,41,5,6,12,13,13a-octahydro- 1H-indolo[3,2,1-de]pyrido[3,2,1-ij][1,5]naphthyridine-12- carboxylate
methyl (41S,12R,13aR)-13a-ethyl-12-
hydroxy-2,3,41,5,6,12,13,13aoctahydro-
1H-indolo[3,2,1-
de]pyrido[3,2,1-ij][1,5]naphthyridine-
12-carboxylate
(3alpha,16alpha)-eburnamenine-14-carboxylic acid
ethyl apovincaminate
|