|
|
| | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI) Basic information |
| Product Name: | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI) | | Synonyms: | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI);2-Thiazolecarboxaldehyde, 4,5-diMethyl-;4,5-Dimethyl-1,3-thiazole-2-carboxaldehyde;4,5-Dimethylthiazole-2-carboxaldehyde;4,5-Dimethyl-2-formylthiazole;4,5-Dimethyl-2-thiazolecarbaldehyde;4,5-DiMethylthiazole-2-carbaldehyde;4,5-Dimethyl-2-thiazolecarboxaldehyde | | CAS: | 74531-15-0 | | MF: | C6H7NOS | | MW: | 141.19 | | EINECS: | | | Product Categories: | ALDEHYDE | | Mol File: | 74531-15-0.mol | 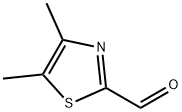 |
| | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI) Chemical Properties |
| Melting point | 33-39°C | | Boiling point | 260℃ | | density | 1.213 | | Fp | 111℃ | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | Appearance | Light yellow to yellow Solid |
| Hazard Codes | Xn | | Risk Statements | 22-36 | | Safety Statements | 26 | | WGK Germany | 3 | | HS Code | 2934100090 |
| | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI) Usage And Synthesis |
| Synthesis | Under argon protection, 4,5-dimethylthiazole (600 μL, 5.5 mmol) was dissolved in anhydrous tetrahydrofuran (40 mL) and cooled to -78 °C. n-Butyllithium (2.3 M hexane solution, 3.6 mL, 8.28 mmol) was slowly added and the reaction mixture was stirred at -78 °C for 1 hour. Subsequently, a solution of anhydrous tetrahydrofuran (10 mL) in N,N-dimethylformamide (1.1 mL, 14.2 mmol) was added. The resulting mixture was stirred at a temperature range of -78 °C to -60 °C for 2.5 hours. Upon completion of the reaction, the reaction was quenched by the addition of acetic acid (0.5 mL) and aqueous ammonium chloride and the mixture was allowed to warm up to room temperature. The reaction mixture was extracted with ether and ethyl acetate, the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated to obtain the crude product. Purification of the crude product by fast column chromatography (silica gel, eluent cyclohexane/ethyl acetate, ratio tapered from 1/0 to 7/3) afforded 4,5-dimethyl-1,3-thiazole-2-carboxaldehyde (724 mg, 93% yield), which was initially a yellow oil that was converted to a white solid after storage at -18 °C. ESI-MS m/z 160 ([M + H2O + H]+). | | References | [1] Patent: EP2141164, 2010, A1. Location in patent: Page/Page column 10-11
[2] Patent: EP2080761, 2009, A1. Location in patent: Page/Page column 39
[3] Patent: WO2013/149362, 2013, A1. Location in patent: Page/Page column 48-49
[4] Patent: WO2014/139150, 2014, A1. Location in patent: Page/Page column 76; 77
[5] Patent: WO2014/150114, 2014, A1. Location in patent: Page/Page column 76; 77 |
| | 2-Thiazolecarboxaldehyde, 4,5-dimethyl- (6CI, 9CI) Preparation Products And Raw materials |
|