|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アルブテロール | | 英語化学名: | Salbutamol | | 别名: | sultanol;Albuterol, α-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-α,αμ-diol;4-Hydroxy-3-hydroxymethyl-α-[(tert-butylamino)methyl]benzyl alcohol;rac-(R*)-4-Hydroxy-3-(hydroxymethyl)-α-[[(tert-butyl)amino]methyl]benzyl alcohol;(S)-1-(4-Hydroxy-3-hydroxymethylphenyl)-2-(1,1-dimethylethylamino)ethanol;(S)-1-(4-Hydroxy-3-hydroxymethylphenyl)-2-tert-butylaminoethanol;(S)-4-Hydroxy-3-(hydroxymethyl)-α-[[(tert-butyl)amino]methyl]benzyl alcohol;2-(Hydroxymethyl)-4-[(S)-1-hydroxy-2-[(1,1-dimethylethyl)amino]ethyl]phenol | | CAS番号: | 18559-94-9 | | 分子式: | C13H21NO3 | | 分子量: | 239.32 | | EINECS: | 242-424-0 | | カテゴリ情報: | CELLCEPT;Steroids & Hormones - 13C & 2H;11 | | Mol File: | 18559-94-9.mol | 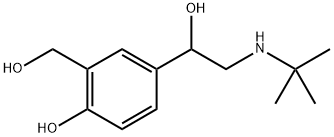 |
| | アルブテロール Usage And Synthesis |
| 用途 | サルブタモール(Salbutamol)は短時間作用性β2アドレナリン受容体刺激剤であり、喘息や慢性閉塞性肺疾患における気管支痙攣のリリーフに使われる。米国ではアルブテロール(米国一般名Albuterol)と呼ばれる。
サルブタモールは世界中でもっともよく処方されている気管支拡張剤であり、吸入(定量噴霧式吸入器、ネブライザー)、経口(錠剤、シロップ)の形で投薬される。
サルブタモールは気管支平滑筋のほかに、子宮平滑筋も弛緩させるため、早産防止のために静脈注射で投与されることもある(日本では用法外)。また、β刺激剤の副作用として脂肪燃焼、骨格筋増強の効果があり、海外ではダイエットやボディビルディングの目的でサルブタモールの錠剤が使用されることもある。 | | 効能 | 気管支拡張薬, β2アドレナリン受容体作動薬 | | 説明 | Albuterol is a β2-adrenergic sympathomimetic amine with pharmacological similarities to
terbutaline. It has almost no effect on β1-adrenoreceptors of the heart. It has expressed
broncholytic effects—prevention or relief of bronchi spasms, lowering respiratory tract
resistance, and increasing the vital capacity of the lungs. | | 化学的特性 | solid | | Originator | Ventolin ,Allen and Hanburys ,UK ,1969 | | 使用 | Albuterol is widely used for severe and chronic bronchial asthma and other illnesses of the res�piratory tract that result in a spastic condition of the bronchi. | | 使用 | short-acting b2-adrenergic agonist, asthma therapeutic | | 使用 | immune suppressant, antineoplastic, antiviral | | 定義 | ChEBI: A member of the class of phenylethanolamines that is 4-(2-amino-1-hydroxyethyl)-2-(hydroxymethyl)phenol having a tert-butyl group attached to the nirogen atom. It acts as a beta-adrenergic agonist used in the treatment of asthma
and chronic obstructive pulmonary disease (COPD). | | Manufacturing Process | (a) α1-Benzyl-tert-butylaminomethyl-4-hydroxym-xylene-α1,α3-diol: 3.0 g of
5-(N-benzyl-N-tert-butylglycyl)-salicylic acid methyl ester hydrochloride in 40
ml of water was basified with sodium bicarbonate solution and extracted into
ether. The ethereal solution was dried over MgSO4 and evaporated and the
basic residue in 20 ml of dry tetrahydrofuran was added with stirring to 1.0 g
of lithium aluminum hydride in 100 ml of dry tetrahydrofuran, over a period of
5 minutes. The light gelatinous precipitate that formed was stirred and
refluxed for 8 hours after which time 7 ml of water was carefully added and
the solvents were removed under reduced pressure.
The residue was acidified with dilute hydrochloric acid and brought to pH 8
with sodium hydroxide and sodium bicarbonate. The mixture was filtered and
the filtrate and orange solid were separately extracted with chloroform. The
combined, dried, chloroform solutions were evaporated to give 22 g of the
crude basic triol as an orange solid, when triturated with ether. A portion of
the material was recrystallized from ether/light petroleum (BP 40-60°C) to
give a white solid, MP 109-111°C.
In an alternative process, sodium borohydride was used as the reducing
agent, as follows:
36 g of 2-(benzyl-tert-butylamino)-4'-hydroxy-3'-hydroxymethyl
acetophenone, hydrochloride was shaken with 100 ml of 10% sodium
carbonate solution and 100 ml of ethyl acetate. The ethyl acetate layer was
separated, washed with water, dried over anhydrous sodium sulfate and
evaporated in vacuum.
The residual gum was dissolved in 360 ml of ethanol and cooled to 15°C in an
ice/water bath, 8 g of sodium borohydride was then added in portions over 30
minutes while maintaining the temperature at 15-20°C. After a further 30
minutes at 20°C the solution was stirred at room temperature for 2 hours.
The solution was again cooled in ice and 250 ml of 2 N sulfuric acid were
slowly added, then the solution was evaporated in vacuum until the ethanol
had been removed. The clear aqueous solution was then treated with 250 ml
of 10% sodium carbonate solution and the oil which precipitated was
extracted into ethyl acetate. The ethyl acetate layer was washed with sodium
carbonate solution, then with water, and was dried over anhydrous sodium sulfate and evaporated in vacuum, to a small volume. Petroleum ether (BP
40-60°C) was added, and after standing overnight a white solid was obtained.
This was filtered off to give 23 g of the product, MP 110-114°C.
(b) α1-tert-Butylaminomethyl-4-hydroxy-m-xylene-α1,α3-diol: 0.8 g of α1-
benzyl-tert-butyl-aminomethyl-4-hydroxy-m-xylene-α1,α3-diol in 20 ml of
ethanol and 2 ml of water was shaken with hydrogen in presence of 0.50 g of
pre-reduced 10% palladium on charcoal catalyst. When uptake of hydrogen
was complete, the solution was filtered and evaporated under reduced
pressure to give 0,4 g of the base as a colorless oil which yielded a white
solid, MP 144-145°C when triturated with ether/cyclohexane. Recrystallization
from ethyl acetate-cyclohexane gave a white solid, MP 147-149°C. | | brand name | Proventil (Schering);
Ventolin (GlaxoSmithKline). | | Therapeutic Function | Bronchodilator | | 生物学の機能 | Levalbuterol is the R-(–)-isomer of albuterol and is available only in solution to be administered via nebulizer. Because it is the active isomer, the dose is fourfold less than that of albuterol. Pirbuterol is the pyridine isostere of albuterol. It has pharmacokinetics similar to albuterol but is half as potent at the β2-receptor. Pirbuterol is only available as an inhaler, whereas albuterol comes in tablet, syrup, solution, and aerosol formulations. | | Synthesis Reference(s) | Synthesis, p. 966, 1988 DOI: 10.1055/s-1988-27768 | | 一般的な説明 | Standard for Supelco MIP SPE cartridges. For more information request Supelco Literature T407075, T706019, T706030, T706020. Salbutamol is classified under the β-agonist group of chemicals which are known to possess powerful pharmacological activities. | | Biochem/physiol Actions | β2-adrenoceptor agonist | | 臨床応用 | Albuterol has the N-t-butyl and a salicyl alcohol phenyl ring, which gives it optimal β2-selectivity. It is resistant to COMT and slowly metabolized by MAO, giving it good oral bioavailability. Its onset by inhalation is within 5 minutes, with a duration of action between 4 and 8 hours. It currently is the drug of choice for relief of the acute bronchospasm of an asthmatic attack. | | 副作用 | Adverse effects of pirbuterol are nervousness, tremor, and headache, which is less than the profile for albuterol, which adds nausea, vomiting, dizziness, hypertension, insomnia, tachycardia, and palpitations. | | 合成 | Albuterol, 2-tert-butylamino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol
(11.1.26), basically differs from all of the aforementioned sympathomimetics in that the
hydroxyl group at C3 of the aromatic ring is replaced with a hydroxymethyl group. It is
synthesized in two ways. According to the first, it is prepared from 4-hydroxyacetophe�none, the chloromethylation of which gives 4-hydroxy-3-hydroxymethylacetophenone
(11.1.20). This is acetylated into a diacetyl derivative (11.1.21), which is further bromi�nated into the corresponding bromoacetophenone (11.1.22). Reacting this with N-benzyl�N-tert-butylamine gives a derivative of aminoacetophenone (11.1.23), the acetyl group of
which is hydrolyzed by hydrochloric acid, and the resulting product (11.1.24) undergoes a
reduction?afirst by sodium borohydride for transforming the keto group into a hydroxyl
group to give 11.1.25, and then by hydrogenation over a palladium catalyst for removing
the benzyl-protecting group, giving albuterol (11.1.26) [26¨C30]. 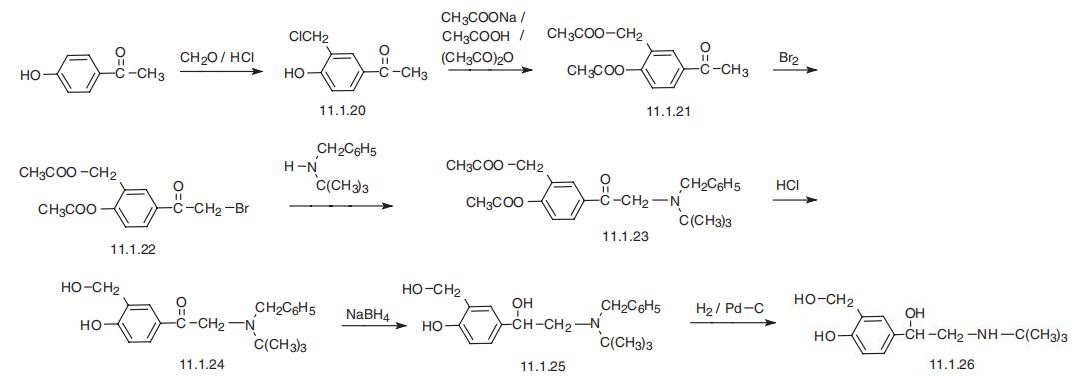
| | 環境運命予測 | Tachycardia occurs as a reflex to the drop in mean arterial
pressure (MAP) or as a result of b-1 stimulus. b-Adrenergic
receptors in the locus coeruleus also regulate norepinephrineinduced
inhibitory effects, resulting in agitation, restlessness,
and hand tremor. Stimulation of nonpulmonary b2 receptors
may lead to an increase in heart rate, QTc interval prolongation,
nonspecific T-wave changes, skeletal muscle tremor, and slight
increases in blood glucose and nonesterified fatty acids. Hypokalemia
is more pronounced in patients receiving intravenous
albuterol. Hypotension is also known to occur mostly in overdose.
The buildup of cyclic AMP in the liver stimulates glycogenolysis
and an increase in serum glucose.
In skeletal muscle, this process results in increased lactate
production. Direct stimulus of sodium/potassium ATPase in
skeletal muscle produces a shift of potassium from the extracellular
space to the intracellular space. Relaxation of smooth
muscle produces a dilation of the vasculature supplying skeletal
muscle, which results in a drop in diastolic and MAP.Myocardial ischemia and infarction have been associated with
excessive tachycardia in elderly patients. The skin may be warm
and pink with evidence of diaphoresis. | | Toxicity evaluation | Albuterol’s production and use as a bronchodilator may result
in its release to the environment through various waste streams. |
|