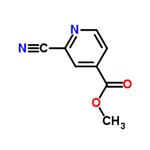
- Methyl 2-cyanoisonicotinate
-
- $30.00 / 1KG
-
2025-09-25
- CAS:94413-64-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | 2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER Basic information | | Uses |
| Product Name: | 2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER | | Synonyms: | Methyl 2-cyanoisonicotinate;Methyl 2-cyanopyridine-4-carboxylate;2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER;Methyl 2-cyanois;4-Pyridinecarboxylicacid, 2-cyano-, Methyl ester;2-Cyano-4-carbomethoxypyridine;F2-Cyano-4-Pyridine Carboxylic Acid Methyl Ester;Methyl 2-cyano-4-pyridinecarboxylate | | CAS: | 94413-64-6 | | MF: | C8H6N2O2 | | MW: | 162.15 | | EINECS: | | | Product Categories: | | | Mol File: | 94413-64-6.mol | 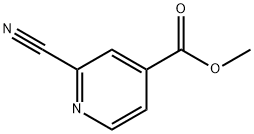 |
| | 2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER Chemical Properties |
| Melting point | 107-109℃ | | Boiling point | 296.6±25.0 °C(Predicted) | | density | 1.25±0.1 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | pka | -2.43±0.10(Predicted) | | form | Solid | | color | Pale brown | | Water Solubility | Slightly Soluble in water (6.2 g/L) (25°C). | | InChI | InChI=1S/C8H6N2O2/c1-12-8(11)6-2-3-10-7(4-6)5-9/h2-4H,1H3 | | InChIKey | ORVHMLCJEKDDAX-UHFFFAOYSA-N | | SMILES | C1(C#N)=NC=CC(C(OC)=O)=C1 |
| | 2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER Usage And Synthesis |
| Uses | 2-Cyano-4-pyridinecarboxylic Acid Methyl Ester, can be used in the synthesis of various pharmaceutical and biologically active compounds, such as tetrasubstituted imidazolines as potent and selective neuropeptide Y (NPY) Y5 receptor antagonists. | | Uses | Methyl 2-cyanoisonicotinate can be used in the synthesis of various pharmaceutical and biologically active compounds, such as tetra substituted imidazolines as potent and selective neuropeptide Y (NPY) Y5 receptor antagonists. | | Synthesis | General procedure for the synthesis of methyl 2-cyano-4-pyridinecarboxylate from zinc cyanide and methyl 2-bromopyridine-4-carboxylate: methyl 2-bromopyridine-4-carboxylate (17.4 g, 80.5 mmol) was dissolved in N,N-dimethylformamide (DMF, 160 mL), and then zinc cyanide (Zn(CN)2, 5.7 g, 48.53 mmol) was added in a single step . The reaction mixture was degassed with nitrogen followed by the addition of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4, 4.7 g). The mixture was again degassed and then the reaction was heated in an oil bath at 120 °C. After 2.5 hours of reaction, the reaction mixture was cooled to room temperature and water (200 mL) was added. The mixture was stirred for 30 minutes and subsequently filtered through a glass sand core funnel. The collected solid was washed with distilled water (2 x 100 mL) and then dried under vacuum to afford methyl 2-cyano-4-pyridinecarboxylate in 84% yield (11.0 g, 67.9 mmol). Electrospray mass spectrometry (ESI-MS) analysis showed [M + H]+ m/z 163.2 (C8H5N2O2 + H, calculated value: 162.1). The obtained product, methyl 2-cyano-4-pyridinecarboxylate, can be directly used in the subsequent reaction without further purification. | | References | [1] Patent: US9139612, 2015, B2. Location in patent: Page/Page column 153; 154; 159
[2] Patent: US2017/100390, 2017, A1. Location in patent: Paragraph 1104
[3] Patent: WO2018/170465, 2018, A1. Location in patent: Paragraph 248 |
| | 2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER Preparation Products And Raw materials |
|